International Journal of Biomedical Science
1(1), 23-32, Jun 15, 2005
© 2005 Master Publishing Group
The effects of Rheum tanguticum polysaccharide on the polarization of Th1 and Th2 cells in TNBS-induced colitis in murine
Liu Li1, Mei Qi-Bing*1,
Wang Zhi-Peng1,
Zhou Yu-Mei1, Zhang Hua2,
Long Yin1, Liu Jia-Yun1
1 Department of Pharmacology, Fourth Military Medical
University, Xi'an 710032, China
2 North China Pharmaceutical Group
Corporation, Shijiazhuang, 050015, China
|
|
ABSTRACT
 |
Inflammatory bowel diseases (IBDs) are chronic inflammatory intestinal disorders
that are characterized by thickened colon walls, colon ulcerations, including
two forms of ulcerative colitis (UC) and Crohn's disease (CD). UC and CD share
some similar clinico-pathological characteristics but their causes are opposite.
The imbalance in cytokinesis produced by Th1 and Th2 cells, the subunit of CD4+
T cells, plays a key role in the development of IBDs. Although traditional
treatment for IBDs is effective to some patients, it has numerous
adverse-effects such as immuno-depression. In our previous study we found some
therapeutic effects of Rheum tanguticum polysaccharide (RTP) on CD. Our present
investigation focuses on the comparison of the effects of RTP (200 mg•kg-1, once
a day for five days) on UC induced by trinitrobenzene sulfonic acid
(TNBS)/ethanol in BALB/c mice and CD induced by TNBS in SD rats. The mechanism
of RTP was investigated by using immuno-histochemistry, Elisa assay, flow
cytometry and western-blot analysis. Our results showed that RTP had significant
therapeutic effects on both UC and CD. The ulcerative index and colon weight
were significantly attenuated after RTP treatment while MPO activity in
RTP-treated animals was markedly lower than that in the animals of TNBS
administration (P﹤0.05, P﹤0.01). RTP also showed significant inhibitory effects
on the expansion of CD4+T cells simultaneously improving the imbalance of Th1
and Th2 polarization (P﹤0.01). In conclusion, RTP appears to poses all the
pre-requisites to be applied in therapeutic intervention, thus, offering a hope
for effective treatment for IBDs.
Abbreviation: Crohn's disease (CD), gas chromatography (GC),
cocorticosteroids (CS), inflammatory bowel diseases (IBDs), interleukins
(ILs),myloperoxidase (MPO), Rheum tanguticum Polysaccharide (RTP),
2,4,6-trinitrobenzene sulfonic acid (TNBS), trifluoroacetic acid (TFA)
,ulcerative colitis (UC)
|
|
INTRODUCTION
 |
Inflammatory bowel diseases(IBDs),including ulcerative colitis (UC) and Crohn's
disease(CD), are complex immune-mediated diseases characterized by chronic
inflammation of the intestine[1]. In recent decade, UC and CD
were considered as more frequently-occurring disease placing big burden on
patients and people with sedentary habits (increasingly observed in office
personnel). With a prevalence of about 0.1% in many developed countries, the
incidence of the disease has increased rapidly in China. It is reported that
inappropriate CD4+T cell response in mucosa immunity plays an important role in
IBDs [2]. In addition, the imbalance in cytokines produced by
Th1 and Th2 cells, the subunit of CD4+ T cells, plays a key role in the
development of IBDs [3]. Although UC and CD show some similar
clinico-pathological characteristics, their causes are opposite
[4]. Growing evidence has suggested that in humans, there is an excessive
Th1 cell response associated with excessive IFN-γand IL-12 secretion in CD
cases, whereas excessive Th2 cell response is associated with excessive IL-4 and
IL-10 secretion in UC cases [1]. The most common conventional
therapy for both UC and CD in clinical practice is the use of anti-inflammation
drugs including steroidal and non-steroidal anti-inflammatory drugs, such as
5-amino salicylic acid and glucocorticosteroids (GCs). Although both of them are
very effective in short term, they show some side effects, especially GCs have
some immunosuppressive effects, which restrict their clinical use
[5]. Chinese people have used rhubarb as a folk remedy for gastrointestinal
disease for two thousand years. Some traditional Chinese decoction contains
rhubarb, which has some significant therapeutic effects on both CD and UC in
clinic. In our previous studies, the polysaccharide extracted from Rheum
tanguticum, one breed of rhubarb, showed striking protective effects on CD
induced by TNBS in rats [6]. However, there is no report about
the effects of Rheum tanguticum polysaccharide (RTP) on UC and the therapeutic
mechanism of RTP on CD and UC is still unclear. Elson et al [7]
once reported that the characteristics of UC and CD could be illustrated by
intestinal inflammatory models induced by trinitrobenzene sulfonic acid (TNBS)
in different animal strains. Our present study is based on his reports to study
the effects of RTP on CD and UC (CD was induced by TNBS/ethanol in SD rats while
UC was induced in BALB/c mice) and to explore the effects of RTP on the
expansion of CD4+T cells and the polarization of Th1 and Th2 cells.
|
|
MATERIALS
AND METHODS |
1.1 Animals
Adult Sprague-Dawley (SD) rats, weighing 220g to 250g, and adult BALB/c mice,
weighing 18g to 20g, were obtained from the Animal Center of the Fourth Military
Medical University and the animals used in the experiment were approved by the
Institutional Ethical Committee of the Fourth Military Medical University. They
were fed with a standard laboratory diet and tap water ad libitum and kept in a
room with controlled temperature (22 ± 1°C), humidity (50 – 70%), and a 12:12h
light-dark cycle. The total number of animals used three times in the experiment
was 34 rats and 57 mice, which were divided into three groups respectively,
including saline, TNBS alone, and TNBS + RTP.
1.2 Regents
2,4,6-trinitrobenzene sulfonic acid (TNBS), fucose, galacturonic, galactose,
mannos, arabinose, sorbose, glucose and glucuronic standard monosaccharide,
ethylenediaminetetraacetic acid (EDTA), dextrans, xanthine and xanthine oxidase
from Sigma (St. Louis, MO, USA), hexadecytrimethyl-ammonium from Fluka (Buchs,
Switzerland) were used in the experiment. CD4 Mab was purchased from Immunotech
Company, and INF-γ and IL-4 were obtained from BioSource International
(Camatillo, CA, USA).
1.3 Preparation of polysaccharide from R tanguticum
Rhubarb was identified as Rheum tanguticum Maxim. ex Regel Tolygonaceac by
Professor Ren Yi in Northwest University in Xi'an, China. Rheum tanguticum
polysaccharide (RTP) was extracted according to the methods described previously
[8]. In brief, Rheum tanguticum Maxim.ex Regel (1kg) was
fragmented and boiled with ethanol to remove the components dissolved in
ethanol. The residue was boiled with water to extract polysaccharides. The
polysaccharide-enriched fractions were obtained by precipitation with 5 volumes
of ethanol 5 times. After the proteins were removed with freezing-thawing and
savage methods, 7-gram of crude Rheum tanguticum polysaccharide from per
100-gram rhubarb was obtained by dialysis (molecular weight cut-off 8000) with
distilled water for 72 hours, by concentration and by lyophilization. The gel
column (5cm ×100cm) was packed with Sephacryl-S400 and eluted with 0.1N NaCl at
a flow rate of 2ml/min at room temperature. 10ml elute was collected in a
Pharmacia LKB Superfrac fraction collector. Then the polysaccharide contents
were determined by the phenol sulphuric acid assay, and the uronic acid contents
were used with colorimetrical assay. The molecular weight of RTP was identified
by gel chromatography, in which the gel column (1cm×80cm) was packed with
Sephacryl-S400 and eluted with 0.1N NaCl at a flow rate of 1ml/min at room
temperature. The molecular weight of RTP was 60-80kDa, which was calculated from
the standard MW-eluted volume relationships that were generated with five known
different MW dextrans (2x105, 3x105, 5x105, 6x105, and 8x105). Monosaccharide
composition was identified by gas chromatography (GC) (Shimadzu, Japan) with
flame ionisation detector (Hewlett-Packard, Vienna, Austria). RTP was composed
of galactose, galacturonic, arabinose, glucose, glucuronic and sorbose.
1.4 Induction of experimental inflammatory bowel disease in murine
The experimental CD in SD rats and UC in BALB/c mice were induced with reference
to what Elson et al [7] described, respectively. The animals
were fasted for 24 hours before experimentation but had free access to drinking
tap water. After the animals were slightly anesthetized with diethyl ether, TNBS
(30mg TNBS/40% ethanol to rats, 3mg TNBS/38% ethanol to mice) was slowly
injected into the colon through the rectum using a polyethylene catheter (10cm
long and 2mm external diameter for rats, 6cm long and 1mm external diameter for
mice). RTP was orally administrated to the animals once a day for 5 days and
then they were sacrificed on 6th day after RTP treatment. The distal colon was
removed, opened and rinsed thoroughly in ice-cold saline. The lesion area was
measured using a 1mm2 grid by a single observer who was blind to treated and
untreated animals. The distal colons with the length of 8cm of rats and 4cm of
mice were weighted. The ratio of colon weight to body weight was used to assess
the degree of colon edema, which reflected the severity of colonic inflammation.
The samples were excised, and the colonic mucosa was carefully scraped off with
a glass slide and snap-frozen in liquid nitrogen, and stored at -80°C to further
assay myloperoxidase (MPO) activities within a week. Then the spleen and thymus
were removed and weighted.
1.5 Measurement of myeloperoxidase (MPO) activity in the colonic mucosa
MPO activity was determined with a modified method described by Krawisz et al
[9]. In brief, 50mg colonic mucosa was homogenized with a
homogenizer for 40s in an ice-cold 50mM phosphate-buffered saline solution
containing 0.5% hexadecyl- trimethylammonium bromide (pH 6.0). The homogenate
was freeze-thawed three times, followed by repeated sonication for 30s. The
supernatant was collected after centrifugation (11,000g, 4°C, 20min). The change
in absorbance at 460nm was measured spectrophotometrically from 30s to 90s, and
MPO activity was defined as degrading 1μmol of hydrogen peroxide per minute at
25°C and expressed as unit per gram of tissue.
1.6 Immunohistochemical analysis on CD4+ T lymphocyte
Immuno-histochemical staining was performed on paraffin-embedded sections by
using biotinylated anti-CD4 mAb according to SBC immuno-histochemical kit
procedure.
1.7 Flow cytometry on peripheral blood CD4+ lymphocyte
500μl peripheral blood was taken from caudal vein of SD rats and BALB/c mice,
and was diluted by PBS. Immuno-fluorescence labeling was done with anti-CD4. The
sample was centrifuged three times at 1,100g for 5min. This procedure was
followed by haemolysis, which was achieved by short incubation with 0.83% NH4Cl
pH 7.2. The cells were resuspended in 0.1% formaldehyde solution. Finally, flow
cytometry was performed with Beckman FASC.
1.8 Western-blot analysis on CD4+ lymphocyte
SD rats and BALB/c mice with IBDs induced by TNBS/ethanol were sacrificed. The
lumenal lymphoid node was separated and homogenized in 1ml lysis buffer in ice
bathing for 10 min, then centrifuged at 11,000g for 5min at 4℃, and 30μl
suspension was moved to 15ml lodding buffer. The samples were boiled for 10min
and subjected to electrophoresis on 120g/L SDS-acrylamide gel. The gel was then
electroblotted onto ultrocellulose membrane, and the detection of CD4 protein
was conducted by using anti-CD4 mAb.
1.9 Measurement of cytokine production
SD rats and BALB/c mice with IBDs induced by TNBS/ethanol were sacrificed and
their mesenteric lymph nodes were removed. Lymph nodes were free of adipose
tissue and gently pressed through a wire mesh. Lymphocytes were collected by
centrifugation (1,000g, 10min) and washed twice with RPMI culture medium.
Lymphocytes (5x106) were cultured in 24-well flat bottomed culture plates in RPIM
culture medium containing 10% (v/v) FCS. After 24h the plates were centrifuged
and the culture medium was carefully removed. Cytokine concentrations were
measured by using Elisa assay according to manufacturer's instruction.
1.10 Statistical analysis
The SPSS software was used to determine the statistical significance of the
difference. Results were expressed as mean ± standard error. Differences between
the two groups were examined using the Student's t-test. Mortality was examined
using c2-test. P value with less than 0.05 was considered significant.
|
|
RESULTS
 |
2.1 Effects of RTP on TNBS-induced IBDs in murine
The mortality of BALB/c mice, nearly 70%, was much higher than that of SD rats.
The inflammation in BALB/c mice was primarily limited to the colon, whereas the
inflammation in SD rats was throughout the gastrointestinal tract and much more
severe. The colon damage in SD rats was related to Th1 cell response, which
enhanced the immune response so the inflammatory response became obvious.
However, Th2 cell response depressed the immune response so the inflammation
healing was promoted. RTP presented striking therapeutic effects on TNBS-induced
inflammatory bowel diseases in both SD rats and BALB/c mice (Table1, Figure 1).
View this table:
[in a new window] |
Table 1. Effects of RTP on survival rate in TNBS-induced
IBDs in murine
|
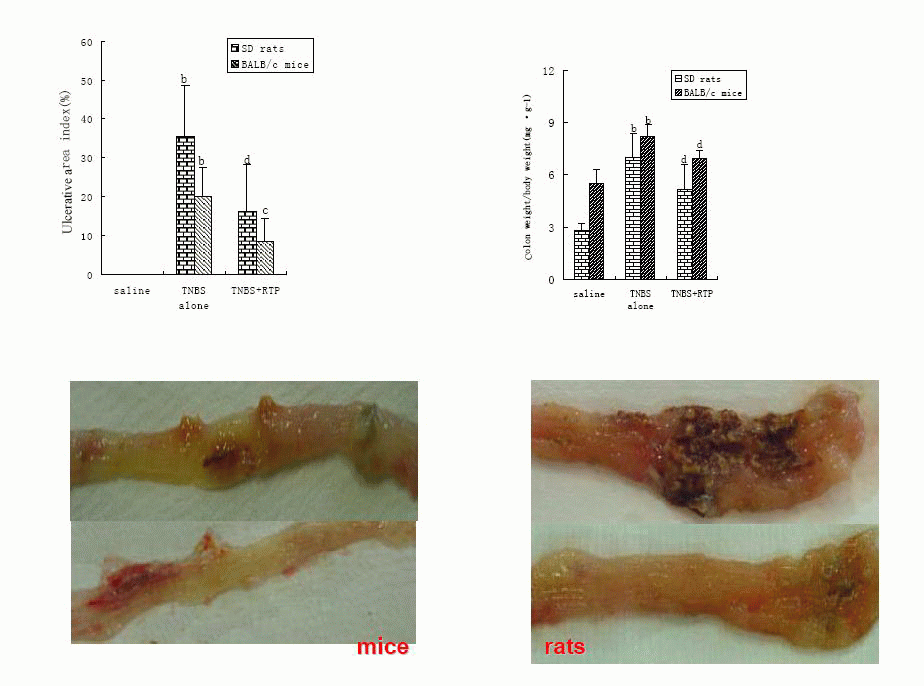
View larger version :
[in a new window] |
Fig. 1. Effects of RTP on TNBS-induced IBDs in murine(n = 10 ,
 ± s)
Animals were administrated with saline, TNBS in ethanol (TNBS alone) and RTP
(TNBS + RTP 200 mg•kg-1). The percentage of ulcerative area indicated the
ratio of the ulcerative area to total area of colon at 8cm length in rats
and at 4cm length in mice. Values were means ± standard error for 10 rats and
10 mice in each group. aP<0.05 vs saline,bP<0.01 vs saline;cP<0.05 vs TNBS
alone, dP<0.01 vs TNBS alone; fP<0.01 vs SD rats . ± s)
Animals were administrated with saline, TNBS in ethanol (TNBS alone) and RTP
(TNBS + RTP 200 mg•kg-1). The percentage of ulcerative area indicated the
ratio of the ulcerative area to total area of colon at 8cm length in rats
and at 4cm length in mice. Values were means ± standard error for 10 rats and
10 mice in each group. aP<0.05 vs saline,bP<0.01 vs saline;cP<0.05 vs TNBS
alone, dP<0.01 vs TNBS alone; fP<0.01 vs SD rats .
| |
2.2 Effects of RTP on MPO activity of colonic mucosa in TNBS-induced IBDs
in murine
MPO enzyme was used as a quantitative index of inflammation and a marker of
neutrophil infiltration in the tissue [9]. The MPO activity in
the colonic tissue dramatically increased after TNBS-enema. The MPO activity in
BALB/c mice was much higher than that in SD rats, which partly explained the
cause of higher mortality of BALB/c mice. Treatment with RTP (200mg昸g-1, p.o)
significantly inhibited the increase of MPO activity both in SD rats and BALB/c
mice (Figure 2).
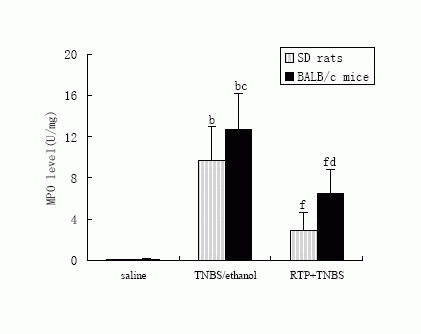
View larger version :
[in a new window] |
Figure 2. Effects of RTP (200mg•kg-1,p.o) on MPO activity of colonic
mucosa in TNBS-induced IBDs in murine.
|
The colonic mucosa MPO activity was measured in murine-received saline, TNBS
in ethanol (TNBS alone) or RTP (TNBS + RTP). Values were means ± standard error
for 10 rats and 10 mice in each group. bP<0.01 vs saline, cP<0.05 vs the same
parameter in SD rats, dP<0.01 vs the same parameter in SD rats; fP<0.01 vs
TNBS/ethanol.
2.3 Effects of RTP on thymus and spleen weight in TNBS-induced IBDs in murine
It was found in the experiment that atrophy of thymus was found in both rats and
BALB/c mice after TNBS-enema, whereas spleen was augment in SD rats, atrophy in
BALB/c mice, which indicated different immune state in IBDs. The mice
administrated with RTP regained spleen weight but RTP did not have any effect on
thymus (Table 2), which was related to the increase of thymus or spleen T cells
in the early stage of inflammation. Then during the inflammation development T
cells migrated to the colon and infiltrated in the local inflammatory site,
which induced the dysfunction of thymus and spleen [10].
View this table:
[in a new window]
|
Table 2. Effects of RTP on thymus and spleen weight in
TNBS-induced IBDs in murine(n = 10 , ±s) ±s)
|
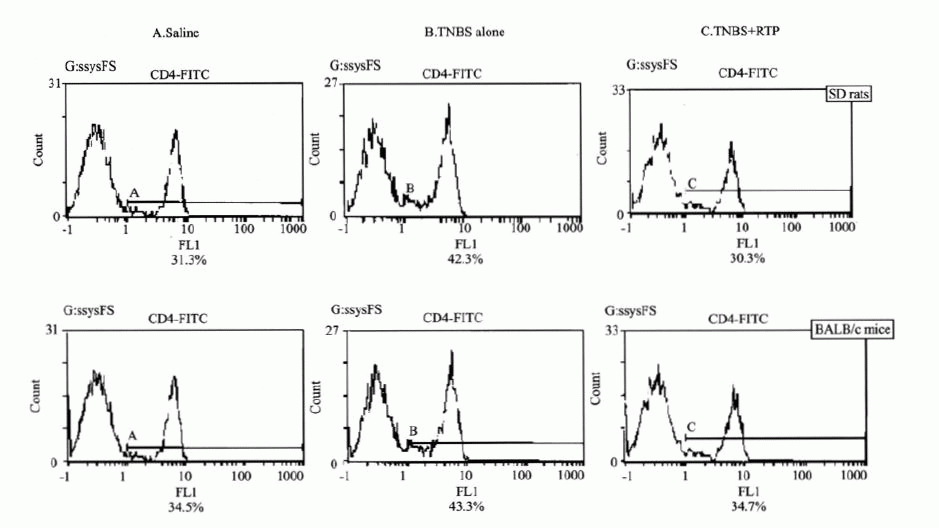
2.4 Effects of RTP on CD4+T cell expansion in TNBS-induced IBDs in murine
Immuno-histochemistry, flow cytometry and western-blot analysis were employed to
investigate the effects of RTP on CD4+T cell expansion. It was found that CD4+T
cell infiltration in colon inflammation, expansion in peripheral blood, and CD4
protein expression up-regulation were showed in animals suffering from
intestinal inflammation and that the resistance of RTP on the expansion of CD4+T
cell was presented significantly in both SD rats and BALB/c mice(Figures 3, 4
and 5).
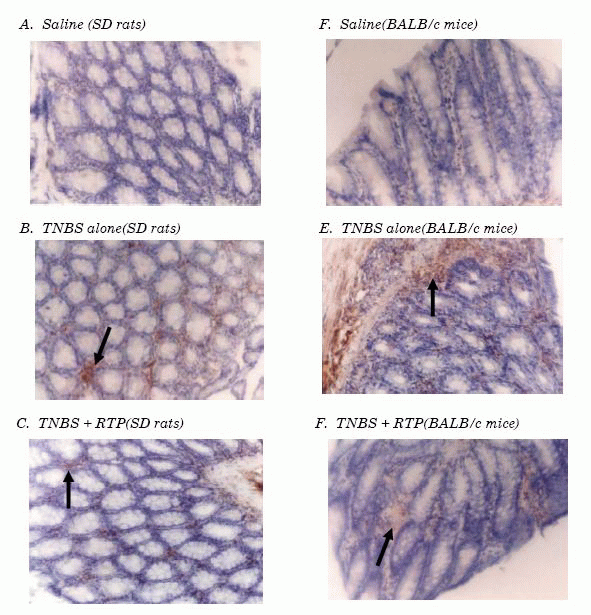
View larger version :
[in a new window] |
Figure 3. Immuno-histochemical analysis of CD4+ lymphocytes isolated
from colon tissue Immuno-histochemical staining of paraffin, colon mucosa
sections from SD rats (A,B and C) and colon mucosa sections from BALB/c mice
( D, E and F). The sections from the animals treated with saline (A and
D),TNBS-induced IBDs (B and E) and RTP treatment (C and F). These photos
stained with CD4 antibody showed positive cells (arrow). (Magnification 400×)
|
View larger version :
[in a new window] |
Figure 4. Flow cytometry on CD4+T cell expansion in peripheral blood
CD4+T cells significantly increased in both SD rats and BALB/c mice treated
with TNBS(B). However, CD4+T cells markedly decreased after RTP
administration (C).
|
View this table:
[in a new window]
|
Table 3. Effect of RTP on CD4+T cell expansion in
peripheral blood(n = 6 ,
 ±s) ±s)
|

View larger version :
[in a new window] |
Figure 5. Western-blot analysis Western-blot analysis of CD4 protein
from the SD rat (Lane A, C and E) and the BALB/c mouse (Lane B, D and F)
lymphoid nude. The lanes from left to right represented the protein isolated
from animals administrated with saline (Lane A and Lane B), TNBS /ethanol
(Lane C and Lane D) and TNBS + RTP( Lane E and Lane F ), respectively.
|
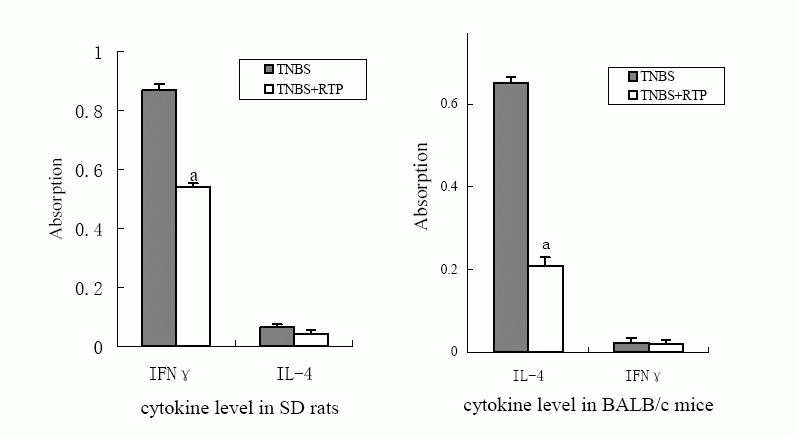
2.5 Effects of RTP on cytokine secreted by Th1 or Th2
cells in TNBS-induced IBDs in murine
IFN-γand IL-4 concentration were measured to investigate the effects of RTP on
cytokine secretion and the polarization of Th1/Th2 in IBD animals treated with
RTP. The product of IFN-γfrom SD rats significantly increased but little amount
of Il-4 product was detected. The marked decrease of IFN-γ product was observed
after RTP treatment. However, Il-4 product from BALB/c mice with UC was much
high, whereas IFN-γproduct was hardly detected. Il-4 product significantly
decreased after RTP treatment in BALB/c mice.
 DISCUSSIONS DISCUSSIONS
 |
In the past few years, a large body of evidence has been accumulated to
suggest that the pathogenesis of IBDs involves genetic susceptibility, immunity
dysfunction, and interaction among local environment microbes
[11]. Crohn's disease (CD) and ulcerative colitis (UC) are together known as
inflammatory bowel disease. CD is characteristic of multifucal and transmural
inflammation throughout the gastrointestinal tract, whereas UC is primarily
limited to the colon. The difference between CD and UC may be related to the
imbalance between Th1 cell response and Th2 cell response [3].
Although CD and UC share some similar clinico-pathological properties, the
causes are opposite. Up to now, there is still no miracle drug to cure the
patients. Glucocorticoid is used as main remedy for IBDs, but the severe adverse
effects, such as immuno-depression, limit its clinical use. It is urgent to
develop new therapeutic strategies. In our previous study, we found Rheum
tanguticum polysaccharide (RTP) showed a striking therapeutic effect on CD of
rats [6]. In our present research, we found some significant
therapeutic effects of RTP on both UC and CD. The failure of TNBS/ethanol to
induce UC in CD4-/- mice indicated that CD4+T cells played a key role in the
development of IBD [12]. It was also found that RTP had some
significant effects on the resistance of the expansion of CD4 +T cells in
periphery blood, the infiltration of CD4 +T cell in colon mucosa, and
up-regulation of CD4 protein expression, which suggested that the mechanism of
RTP on IBDs might be related to modulate CD4+T cell dysfunction. The data
indicated that RTP might have potential therapeutic effects on IBDs.
The IBD animal model induced by TNBS is inexpensive and easy to duplicate so it
is the most common model used to screen anti-IBD drug. Therefore,
TNBS/ethanol-induced animal model is suitable for estimating the therapeutic
effects and mechanism of RTP on IBDs. In 1995, Neurath, et al[13]
reported that different inflammatory characteristics of IBDs were illustrated in
TNBS/ethanol-induced intestinal inflammation animal models in different strains.
The characteristics of Th1 cytokines were similar to human CD, while the
characteristics of Th2 cytokines were similar to human UC. In our present study,
the mucosa inflammation induced by TNBS was more severe in SD rats than that in
BALB/c mice. Five days after TNBS administration to the rats, the colon showed
widespread mucosal necrosis and gross ulceration, whereas the mortality was much
high in BALB/c mice. Th1 cells promoted the development of inflammation and
immune response. However, Th2 cells promoted inflammation healing by depressing
immune response [14]. It was proved in this experiment that
TNBS induced different inflammatory responses in different animal strains. RTP
showed significant effects on the two forms of IBDs (UC and CD) and improved
Th1/Th2 polarization towards the balance of cytokine secretion.
The balance of Th1 and Th2 cells plays an important role in normal immune
response. The abnormal mucosa immune system displays an unbalanced response
consisting of either excessive Th1 response or inadequate Th2 response
[3]. The polarization of Th1 and Th2 cells is affected by some cytokines,
but the mechanism is unclear. Cytokines secreted by Th1 cells, such as TNF-γ and
IL-12, may promote Th1 cell polarization while IL-4 and IL-10 secreted by Th2
cells may promote Th2 cell polarization. Recently, some monoclonal antibody of
cytokines has been found to regulate the imbalance of cytokine. For example, an
antibody of TNF-γ has been used in clinical trial to treat the patients with
moderate or severely active CD.
In conclusion, the results obtained in the present study showed significant
effects of RTP on laboratory animals with UC induced by trinitrobenzene sulfonic
acid (TNBS)/ethanol in BALB/c mice and CD induced by TNBS in SD rats. There was
marked decrease of both IFN-γ in CD rats and IL-4 concentration in UC mice. It
appears that the therapeutic effects of RTP on IBDs are related not only to the
resistance of expansion of CD4+T cells but also to the improvement of the
balance of polarization of Th1 and Th2 cells. Therefore, RTP may be considered
as a potential phyto-therapeutic treatment in IBDs.
|
|
ACKONWLEDGMENTS
 |
This project was supported by the National Nature Science Foundation of
China, No. 30100239, No. 30300450. It was also supported by the Postgraduate
Foundation of North China Pharmaceutical Group Corporation.
|
|
REFERENCES
 |
- 1. Strober W, Fuss IJ, Blumberg S. The immunology
of mucosal models of inflammation. Annu. Rev Immunol 2002; 20: 495-549
- 2. Shanahan F. Crohn's disease. The Lancet 2002;
359: 62 -69
- 3. Tozawa K, Hanai H, Sugimoto K, Baba S, Sugimura
H, Aoshi T. Evidence for the critical role of interleukin-12 but not
interferon-g in the pathogenesis of experimental colitis in mice. J of
Gastroenterology and Hepatology 2003; 18: 578 -587
- 4. Monteleone I, Vavassori P, Biancone L,
Monteleone G, Pallone F. Immunoregulation in the gut: Success and failures in
human disease. Gut 2002; 50: 60 -64
- 5. Shanahan F. Inflammatory bowel disease:
immunodiagnostics, immunotherapeutics and ecotherapeutics. Gastroenterology
2001;120:622- 635
- 6. Liu Li, Mei Qibing, Zhou Siyuan, Han Fenghua,
Long Yin, Liu Jiayun. Effects of Rheum tanguticum on ulcerative colitis induced
by TBNS in rats. China Journal of Chinese Material Medica 2003;28(3):246
-249
- 7. Elson CO, Sartor RB, Tennyson GS, Riddell RH.
Experimental models of inflammatory bowel disease. Gastroenterology 1995;
109(4):1344 -67.
- 8. Cho CH, Mei QB, Shang P, Lee SS, So HL, Guo X,
Li Y. Study of the gastrointestineal protective of polysaccharides from Angelica
sinensis in rats. Planta Med 2000; 66:348 -51
- 9. Krawisz JE,Sharon P,Stenson WF. Quantitative
assay for acute intestinal inflammation based on myeloperoxidase activity.
Gastroenterology 1984; 7: 1344 -1358
- 10. Strober W, Fuss IJ, Ehrhardt RO, Neurath M,
Boirivant M, Ludviksson BR. Mucosal immunoregulation and inflammatory bowel
disease: new insights from murine model of inflammation. Scand J Immunol 1998;
48: 453 - 458
- 11. Romagnani P, Annunziato F, Baccari AM,
Parronchi P. T cells and cytokines in Crohn's disease. Current Opinion in
Immunology 1997;9:793 - 799
- 12. Qiu BS, Vallance BA, Blennerhassett PA,
Collins SM. The role of CD4+ lymphocyte in the susptibility of mice to
stress-induced reactivation of experimental colitis. Nature Medicine 1999;
5(10):1178-1182
- 13. Neurath MF, Fuss I, Kelsall BL, Stuber E,
Strober W. Antibodies to interleukin 12 abrogate established experimental
colitis in mice. J Exp Med 1995; 182:121-129
- 14. Neurath MF, Finotto S, Glimcher LH. The role
of Th1/Th2 polarization in mucosal immunity. Nature Medicine 2002; 8:567 -573
|
|
FOOTNOTES
 |
Address correspondence to Dr. Mei Qi-Bing, Department of Pharmacology, Fourth
Military Medical University. Phone: 86-29-83374552; Fax: 86-29-83374552; E-mail:
pharml@fmmu.edu.cn or qbmei@fmmu.edu.cn



![]() ±s)
±s)