© 2005 Master Publishing Group
Influence of pro- and anti-inflammatory cytokines in Th1 polarization after allogeneic stimulation
Silva R.*, Morgado J.M.*,
Freitas A., Couceiro A., Orfão A.,Regateiro
F.,
Paiva A.
*These authors contributed equally to this work
Histocompatibility Centre of Coimbra
Short Title: Cytokines effects in Th1 polarization
Corresponding author: Artur Augusto Paiva , Histocompatibility
Centre of Coimbra , Edifício São Jerónimo, 4º Piso, Praceta Mota Pinto
, 3030 Coimbra, Portugal
Fax: 00351239480790 Telef: 00351480700 apaiva@histocentro.min-saude.pt
|
|
ABSTRACT
 |
The exogenous cytokine milieu can influence Th1/Th2 polarization. Besides the
differential functional properties, T lymphocytes also acquire distinct profiles
of chemokine receptors. Human Th1 lymphocytes preferentially express CCR5 and
CXCR3 while Th2 lymphocytes express CCR3, CCR4 and CCR8. After their
polarization into Th1 cells, grafted T lymphocytes mediate the development of
graft-vs-host-disease, the major complication after bone marrow transplantation.
We performed mixed lymphocyte cultures for ten days, with and without addition
of IL-2, IL-4, IL-10, IL-12 and IL-18 at the third and sixth day of cultures.
The expression of CXCR3 and CCR5, in CD4+ and CD8+ T lymphocytes was evaluated
by flow cytometry, before and after ten days of culture. The exogenous addition
of IL-2 or IL-12 favoured the Th1/Tc1 phenotype and IL-4 was also capable of
inducing Th1 polarization. In opposition to IL-12, IL-18 didn't induce a
significant polarization into Th1 phenotype, an effect more similar to that
induced by IL-10. This action could explain, at least in part, its possible
protective effect in the incidence of acute and chronic graft-versus-host
disease after allogeneic stem cell transplantation.
Key Words: Flow cytometry; CCR5; Th1/Th2 polarization; GVHD; IL-18
|
|
INTRODUCTION
 |
After activation, T lymphocytes acquire effector functions and can be
subdivided, by a distinct cytokine production, into two subsets. T helper cells
type 1 (Th1) predominantly produce INF-g, IL-2, TNF-b, IL-22
[1 , 2] and IL-24
[3], and mediate a cellular immune response through the activation of
macrophages and cytotoxic T cells. In contrast, Th2 cells predominantly secrete
IL-3, IL-4, IL-5, IL-6, IL-9 and IL-10, potentiate the maturation of B cells and
degranulation of mastocytes, thereby triggering a humoral immune response
[4- 11]. Th1 and Th2 populations differentiate
from naïve T cells after, at least, one round of antigen stimulation. Several
mechanisms can influence Th1/Th2 polarization: the exogenous cytokine milieu,
the nature of the peptide ligand, the activity of some costimulatory molecules
and microenvironmentally secreted hormones
[4- 6, 10,11].
It is actually accepted that Th1 cytokines inhibits the Th2 dominated immune
response and vice versa. Such influence can be demonstrated by the
anti-proliferative effect of INF-g on emerging Th2, and via inhibition of IL-4
and IL-5 dependent B lymphocyte differentiation. IL-4 inhibits Th1 cell
development by down regulating the transcription factors promoting IFN-g
synthesis [4- 6].
Besides the differential functional properties, T lymphocytes also acquire
different activation markers and distinct profiles of chemokines receptors
(CKRs), that together with adhesion molecules (selectins and integrins)
[12] modulate the migration and tissue homing of Th1 and Th2 to
distinct peripheral sites of inflammation, where they can promote different
types of inflammatory reactions [6, 8,
10,13]. Chemokines are chemoattractants, which
direct T cells and other leukocytes into the inflammatory tissues
[6, 14]. Leukocytes respond to chemokines through
specific G-protein-coupled receptors, the chemokine receptors, some of which are
specific and interact with a single chemokine, whereas others are "shared" because
they can bind multiple ligands [14- 16].
There are two major groups for CKRs: CCR (1-10) that binds CC chemokines, and
CXCR (1-5) binding CXC chemokines [17]. Human Th1
and Th2 differentially express chemokine receptors, and therefore their
recruitment is modulated in response to different chemokines
[10, 18, 19]. CCR5 and CXCR3
are preferentially expressed in human Th1 lymphocytes (Th1-associated CKRs),
while Th2 lymphocytes preferentially express CCR3, CCR4 and CCR8 (Th2 associated
CKRs) [9, 10, 18,
19]; CXCR3 is also expressed by Th2 lymphocytes. Because of this
different profile of expression, these receptors could be useful as markers of
Th1/Th2 responses and tools to modulate polarized versions of T cell-dependent
immunity [10].
It has been described [8] that the chemokine
receptor expression on T cells is influenced by the activation state of the
cells as by the cytokines present in the milieu, and correlates with distinct
effector function. For example, while CXCR3 is expressed as a stable marker of
memory Th1 and Th2 cells, CCR5 expression reflects the activation state of the
cells, and it is up-regulated by IL-2 [8,
20]. The expression of CCR5 and CXCR3 are closely linked; all T cells
that express CCR5 also express CXCR3, which may thus be considered an
"opportunistic Th1-associated marker" [18,
19, 21]. This suggests that Th1 lymphocytes may be
chemoattracted through either receptor.
Polarized T cells are involved in specific effector functions and are in
progression of many diseases that also display strikingly polarized pathological
features 21]. It has been suggested that the
balance between Th1/Th2 cytokines is largely determinate of the extent to which
a cell-mediated immune response and a systemic inflammatory response develop
after allogeneic bone marrow transplantation (BMT). The major complication after
BMT is the development of graft-vs-host-disease (GVHD), which is mediated by
grafted T lymphocytes after their polarization into Th1 cells
[22- 24]. Therefore a cytokine capable of inducing
a switch from Th1 to Th2 response inhibiting the production of IL-1 and TNF-a,
may be a new possibility to take in account, with regard to the prevention and
treatment of acute GVHD [22- 24].
In fact, it has been demonstrated that early administration of Th1 inducing
cytokines, including IL-12, IFN-g and IL-2 have shown paradoxical ability to
reduce the severity of acute-GVHD
[25- 27]. Some studies have
failed to demonstrate beneficial effects to direct in vivo administration of Th2
cytokines in preventing or treating acute-GVHD [28,
29].
The aim of this study is to evaluate the influence of anti (IL-4, IL-10), and
pro-inflammatory (IL-2, IL-12 and IL-18) cytokines in the Th1/Th2 polarization
developed during an in vitro allogeneic response with peripheral blood (PB)
mononuclear cells of healthy donors, in order to contribute to a possible
development of novel therapeutic modalities and response to GVHD treatment.
Since in cord blood transplantation (CBT) the incidence and severity of
acute-GVHD seems to be reduced when compared with PB and BM
[29- 34], we also performed our study in human
cord blood samples.
|
|
MATERIALS
AND METHODS
 |
Blood samples
4 human cord blood samples were collected from healthy mothers at normal full
term vaginal deliveries at the Bissaya Barreto Maternity Hospital (Coimbra,
Portugal). The cord blood collections used for this study averaged 50 ml in
volume and were collected to a heparinized container. 5 heparinized peripheral
blood samples were obtained from adult healthy blood donors. Cord blood and
adult peripheral blood mononuclear cells (CBMC and PBMC, respectively) were
isolated by centrifugation over Ficoll-Hypac gradients (LymphoprepTM
Axis shield Pocas, Oslo, Norway). After the isolation, the cells were washed
with Hanks Balanced Salts (HBSS -Gibco, Paisley, Scotland, UK) (15min at 540g),
and then resuspended in 1ml of RPMI-1640 medium (Gibco, Paisley, Scotland UK).
Mixed Lymphocyte Cultures (MLC)
Cord blood mononuclear cells or peripheral blood mononuclear cells responder
cells always presented several HLA mismatched class I and II with stimulator
cells. Each sample of responder cells, at a concentration of 1x106/ml,
supplemented with 10% AB pooled human serum (Sigma, Saint Louis, MO, USA), was
stimulated for 10 days in 96-well microtiter plates with 1x106/ml
allogeneic PBMC cells, treated with mitomycin C (Sigma, Saint Louis, MO, USA).
Cell cultures (4 replicates for each studied cytokine and from each donor) were
incubated at 37ºC in a humidified atmosphere of 5% of CO2, and were
feeded at the 3rd and 6th day, with rhIL-2 (40 U/ml)
(Roche, Mannheim, Germany), rhIL-4 (200 U/ml) (Sigma, Saint Louis, MO, USA),
rhIL-10 (100 U/ml) (PharMingen-BD, San Diego, C.A, USA), rhIL-12 (5 ng/ml) (R&D
Systems, Europe), rhIL-18 (5 ng/ml), (MBL, Naka-ku Nagoya, Japan). Four
replicates were performed without administration of exogenous cytokines.
Chemokine receptors expression
At the 10th day of culture two wells of each combination of the
referred cultures were harvested and centrifuged for 5 minutes at 1500rpm. The
cells were stained for 15 minutes at room temperature in the dark with 10 μl of
each of the specific anti-human MoAbs: CXCR3 FITC (clone 49801; R&D Systems,
Europe), CCR5 PE (clone 2D7; Pharmingen-BD, San Diego, C.A., USA), CD8 PerCP
(clone SK1; BD, San José C.A., USA) or CD4 PerCP (clone SK3; BD, San José C.A.,
USA) and CD3 APC (clone UCHT1; Pharmingen-BD, San Diego, C.A.,USA). After this
incubation period, 2ml of FACS Lysing Solution (BDB) diluted 1:10 (v/v) in
distilled water were added, and the samples were incubated for another 10 min,
under identical conditions, in order to lyse non-nucleated red cells.
Afterwards, cells were centrifuged (5 min, at 540g) and the cell pellet was
washed twice with 2 ml of phosphate-buffered saline (PBS-Dulbecco (1X) - Biochrom
AG, Germany). Finally, cells were resuspended in 0,5 ml of PBS until analyzed in
the flow cytometer.
Flow cytometry data acquisition and analysis
Data acquisition was performed on a FACScalibur flow cytometer (BD, San José
C.A. , USA) equipped with the argon ion laser and a red diode laser. The number
of events acquired for each sample was 10.000 on an electronic CD3+
gate, after a first acquisition of 10.000 of total events.
The identification of the different cell populations was made using "Paint-A-Gate
3.0.2 PPC" software program (BD, San José,USA). T lymphocytes were identified
according to their positivity for CD3 and typical light scatter. Among them, CD4
or CD8 positive T cells were identified according to their reactivity with
anti-CD4 or anti-CD8 monoclonal antibodies. The evaluation of the expression of
these chemokines receptors was evaluated as the percentage of positive cells
within each cell subset and their mean fluorescence intensity (MIF), expressed
as linear fluorescence channels (arbitrary relative linear units scale from 0 to
104).
Statistical analysis
Statistical significance in the difference as observed in the results was
assessed with SPSS 12.0 software using Mann-Whitney U-test or Wilcoxon
signed-rank test , as appropriate.
|
|
RESULTS
 |
Percentage of PB and CB T lymphocytes expressing CXCR3 and CCR5
before allogeneic stimulation
In PBMCs, our results show that CD4 T cells are mainly double negative for CXCR3
and CCR5 [68(36-75)% vs 17(13-37) % and 7,3(0,0-25)% of CXCR3+/CCR5-and
CXCR3+/CCR5+ respectively] while among CD8+ T
cells higher percentages of CXCR3+/CCR5- and CXCR3+/CCR5+
cells are present [42(3.3-77)% and 39(0-67)% respectively] (Fig.1) .
.
On CBMCs almost all CD4+ T cells are negative for CXCR3 and CCR5
[96(93-97)%] while CD8+T cells are, in similar proportions, double
negative [46(5.1-98)%] or CXCR3+/CCR5- [54(2.3-95)%].
Among both populations of T cells no cells simultaneously expressing CXCR3 and
CCR5 were present (Fig.1) . As expected, no CXCR3-/CCR5+ cells were found among
PBMCs or CBMCs.
. As expected, no CXCR3-/CCR5+ cells were found among
PBMCs or CBMCs.
Percentage of PB and CB T lymphocytes expressing CXCR3 and CCR5 after
allogeneic activation
In PBMCs of healthy donors, our results show that the allogeneic stimulation,
without exogenous addition of any cytokine, induced an increase in the
percentage of CD4+ and CD8+ T cells co-expressing CCR5 and
CXCR3 [22(15-51)% and 54(46-65)% of CD4+ and CD8+ T cells
respectively ] (Fig.1)
The cytokines that induce, in a more significant way, the expression of CCR5 in
both, CD4+ and CD8+, T lymphocytes are IL-2 [83(67-88)%
and 95(87-98)% of CD4+ and CD8+
T cells respectively] and IL-12 [66(20-82)% and 84(63-87)% of CD4+
and CD8+ T cells respectively] (Fig.1) . However, in the CD4+ lymphocytes,the percentage of cells expressing
CCR5 is also enhanced in the presence of IL-4 [49(42-63)%] while the increases
that IL-10 and IL-18 induced on CCR5 expression CXCR3 [34(4,4-53)% and
45(5,7-54)% ] are considerably lower than that promoted by IL-2, IL-12 or IL-4
(Fig.1)
. However, in the CD4+ lymphocytes,the percentage of cells expressing
CCR5 is also enhanced in the presence of IL-4 [49(42-63)%] while the increases
that IL-10 and IL-18 induced on CCR5 expression CXCR3 [34(4,4-53)% and
45(5,7-54)% ] are considerably lower than that promoted by IL-2, IL-12 or IL-4
(Fig.1) .
.
With regard to CB T cells, flow-cytometric analysis showed that the allogeneic
stimulus, in the absence of any cytokine didn't induce a significant increase in
the percentage of CD4+ and CD8+ T cells co-expressing CCR5
and CXCR3 [2.1(0-19)% and 0.4(0-17)% of CD4+ and CD8+ T
cells respectively]. IL-12 was the cytokine that induced, in a more significant
way, the expression of CCR5 on the CD4+
subset of T lymphocytes [36(18-61)%]. IL-2, IL-4 and IL-18 also induced the
increase of CCR5 expression [14(7-29)%,11(0-24)% and 9.4(0-22)% respectively],
but in a less significant way. Among CD8+ T cells from CB, the
expression of CCR5 was enhanced by IL-4 [32(0-41)%], IL-12 [27(0-70)%] and IL-18
[11(0-18)%] (Fig.1) .
.
 DISCUSSIONS DISCUSSIONS
 |
The Th1/Th2 polarization of T helper cell subsets may play an important role
in the development of GVHD, a major obstacle to successful allogeneic
hematopoietic stem cell transplantation. The immunopathophysiology of acute-GVHD
is complex, and involves a "cytokine storm" amplified by the Th1 phenotype, which
correlates with the development of acute GVHD. The inhibition of acute-GVHD can
be achieved by a shift to Th2 polarization of donor T cells. Therefore a
cytokine capable of inducing this switch in the donor T cells may be a new
therapeutic agent, with regard to the prevention and treatment of acute-GVHD[22-24,
35]. However, cytokines play a complex and dual role in GVHD, and can
have either protectiveor deleterious effects.
In this study we saw that, in peripheral blood, there are CD4+ and
CD8+ T cells that express CCR5 and, therefore, are already
differentiated into Th1/Tc1 cells (Fig.1) .
.
After activation, T lymphocytes acquire effector functions and differentiate
into Th1 or Th2 cells. In our work, the allogeneic stimulation of PBMCs induced
an increase in the percentage of T cells co-expressing CXCR3 and CCR5, a Th1
phenotype.
Others have described [8, 17,
20] that the expression and responsiveness of certain chemokine
receptors are up-regulated in T cells by stimulation with several cytokines such
as IL-2 and IL-12. In line with the previous findings, after an allogeneic
stimulation in the presence of IL-2 or IL-12, we detected an increase in the
percentage of PB T cells that co-express CCR5 and CXCR3. Moreover, it seems that
IL-4, a Th2 related cytokine, could also have the ability to mediate the
up-regulation of CCR5 expression, at least in the CD4+
subpopulation of T cells, whereas IL-10, promoted a lower induction of CCR5
expression in our study. IL-10 was identified as a cytokine with a dual role; it
has an important anti-inflammatory and immunosuppressive properties but, on the
other hand, has immunostimulatory effects over B and T cells
[36].
The exogenous addition of IL-18 caused a slight increase of CCR5 in CD4+
T cells.
From a functional point of view, IL-18 might be more related to IL-12
[35, 37- 39]
however the role of IL-18 in the differentiation of naive Th cells into Th1
cells is less clear. In the periphery, IL-18 synergistically induces the
expression of the Th1 cytokines in the presence of IL-12 and Th2 cytokines in
the presence of IL-2 [40, 41].
IL-18 alone has minimal effect when compared to IL-18 in combination with IL-12,
in inducing the Th1 cytokine IFN-γ production by tumor-draining lymph node cells
in a murine model [41]. In this regard in
particular, our results show an effect of IL-18 more close to that of IL-10 than
to that of IL-12. IL-18 levels correlate with GVHD course
[42, 43]. Despite reducing the severity of acute
GVHD, preserves the GVL effect after bone-marrow transplantation
[35, 44] and has the remarkable capacity to
modulate acute GVHD when administered either to the donor or the recipient
through distinct mechanisms [45] .
The CCR5 positive cells are almost absent in CD4+ and CD8+
T cell subpopulations of CB. While in PBMCs IL-2 induced Th1 and Tc1
polarization in a similar way, in CBMCs the Tc1 polarization was less pronounced
than Th1. Moreover, the allostimulation with the exogenous addition of IL-2
resulted preferentially in the expression of CXCR3 in the absence of CCR5 than
in the co-expression of both receptors.
These results are in agreement with the naivity that characterizes CB cells and
may, in part, explain the less severity of GVHD associated to cord blood
transplantation.
 |
ACKNOWLEDGEMENTS
 |
The authors wish to thank to the “Comissão de Fomento da Investigação em Cuidados de Saúde - Ministério da Saúde”(Project number 246/99)
|
|
REFERENCES
 |
- 1.Wolk K, Kunz S, Witte E, et al., IL-22 increases the
innate immunity of tissues. Immunity 2004; 21(2): 241.
- 2. Gurney AL, IL-22, a Th1 cytokine that targets the
pancreas and select other peripheral tissues. Int Immunopharmacol 2004;
4(5): 669.
- 3. Chada S, Sutton RB, Ekmekcioglu S, et al., MDA-7/IL-24 is
a unique cytokine--tumor suppressor in the IL-10 family. Int
Immunopharmacol 2004; 4(5): 649.
- 4. Santana MA, Rosenstein Y, What it takes to become an
effector T cell: the process, the cells involved, and the mechanisms. J
Cell Physiol 2003; 195(3): 392.
- 5. Kunzendorf U, Tran TH, Bulfone-Paus S, The Th1-Th2
paradigm in 1998: law of nature or rule with exceptions. Nephrol Dial
Transplant 1998; 13(10): 2445.
- 6. Le Moine A, Goldman M, Abramowicz D, Multiple pathways to
allograft rejection. Transplantation 2002; 73(9): 1373.
- 7. Sadeghi M, Daniel V, Weimer R, et al., Pre-transplant Th1
and post-transplant Th2 cytokine patterns are associated with early
acute rejection in renal transplant recipients. Clin Transplant 2003;
17(2): 151.
- 8. Sallusto F, Lenig D, Mackay CR, et al., Flexible programs
of chemokine receptor expression on human polarized T helper 1 and 2
lymphocytes. J Exp Med 1998; 187(6): 875.
- 9. Loetscher P, Uguccioni M, Bordoli L, et al., CCR5 is
characteristic of Th1 lymphocytes. Nature 1998; 391(6665): 344.
- 10. Bonecchi R, Bianchi G, Bordignon PP, et al.,
Differential expression of chemokine receptors and chemotactic
responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med 1998;
187(1): 129.
- 11. Romagnani S, Th1/Th2 cells. Inflamm Bowel Dis 1999;
5(4): 285.
- 12. Springer TA, Adhesion receptors of the immune system.
Nature 1990; 346(6283): 425.
- 13. Sallusto F, Mackay CR, Lanzavecchia A, Selective
expression of the eotaxin receptor CCR3 by human T helper 2 cells.
Science 1997; 277(5334): 2005.
- 14. el-Sawy T, Fahmy NM, Fairchild RL, Chemokines:
directing leukocyte infiltration into allografts. Curr Opin Immunol
2002; 14(5): 562.
- 15. Cascieri MA, Springer MS, The
chemokine/chemokine-receptor family: potential and progress for
therapeutic intervention. Curr Opin Chem Biol 2000; 4(4): 420.
- 16. Raport CJ, Gosling J, Schweickart VL, et al., Molecular
cloning and functional characterization of a novel human CC chemokine
receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J Biol Chem 1996;
271(29): 17161.
- 17. Sato K, Kawasaki H, Nagayama H, et al., Chemokine
receptor expressions and responsiveness of cord blood T cells. J Immunol
2001; 166(3): 1659.
- 18. Qin S, Rottman JB, Myers P, et al., The chemokine
receptors CXCR3 and CCR5 mark subsets of T cells associated with certain
inflammatory reactions. J Clin Invest 1998; 101(4): 746.
- 19. Inston NG, Cockwell P, The evolving role of chemokines
and their receptors in acute allograft rejection. Nephrol Dial
Transplant 2002; 17(8): 1374.
- 20. Loetscher P, Seitz M, Baggiolini M, et al.,
Interleukin-2 regulates CC chemokine receptor expression and chemotactic
responsiveness in T lymphocytes. J Exp Med 1996; 184(2): 569.
- 21. Kim CH, Rott L, Kunkel EJ, et al., Rules of chemokine
receptor association with T cell polarization in vivo. J Clin Invest
2001; 108(9): 1331.
- 22. Krenger W, Ferrara JL, Graft-versus-host disease and
the Th1/Th2 paradigm. Immunol Res 1996; 15(1): 50.
- 23. Ferrara JL, The cytokine modulation of acute
graft-versus-host disease. Bone Marrow Transplant 1998; 21 Suppl 3(S13.
- 24. Ferrara JL, Cooke KR, Pan L, et al., The
immunopathophysiology of acute graft-versus-host-disease. Stem Cells
1996; 14(5): 473.
- 25. Sykes M, Szot GL, Nguyen PL, et al., Interleukin-12
inhibits murine graft-versus-host disease. Blood 1995; 86(6): 2429.
- 26. Brok HP, Heidt PJ, van der Meide PH, et al.,
Interferon-gamma prevents graft-versus-host disease after allogeneic
bone marrow transplantation in mice. J Immunol 1993; 151(11): 6451.
- 27. Sykes M, Romick ML, Sachs DH, Interleukin 2 prevents
graft-versus-host disease while preserving the graft-versus-leukemia
effect of allogeneic T cells. Proc Natl Acad Sci U S A 1990; 87(15):
5633.
- 28. Krenger W, Snyder K, Smith S, et al., Effects of
exogenous interleukin-10 in a murine model of graft-versus-host disease
to minor histocompatibility antigens. Transplantation 1994; 58(11):
1251.
- 29. Blazar BR, Taylor PA, Smith S, et al., Interleukin-10
administration decreases survival in murine recipients of major
histocompatibility complex disparate donor bone marrow grafts. Blood
1995; 85(3): 842.
- 30. Harris DT, Cord blood transplantation: implications
for graft vs. host disease and graft vs. leukemia. Blood Cells 1994;
20(2-3): 560.
- 31. Risdon G, Gaddy J, Broxmeyer HE, Allogeneic responses
of human umbilical cord blood. Blood Cells 1994; 20(2-3): 566.
- 32. Chargui J, Masao H, Yoshimura R, et al., NK/Cytotoxic
T cells: major effector cells in GVHD after umbilical cord blood
allotransplantation. Transplant Proc 2000; 32(7): 2454.
- 33. Risdon G, Gaddy J, Stehman FB, et al., Proliferative
and cytotoxic responses of human cord blood T lymphocytes following
allogeneic stimulation. Cell Immunol 1994; 154(1): 14.
- 34. Harris DT, LoCascio J, Besencon FJ, Analysis of the
alloreactive capacity of human umbilical cord blood: implications for
graft-versus-host disease. Bone Marrow Transplant 1994; 14(4): 545.
- 35. Reddy P, Teshima T, Hildebrandt G, et al., Interleukin
18 preserves a perforin-dependent graft-versus-leukemia effect after
allogeneic bone marrow transplantation. Blood 2002; 100(9): 3429.
- 36. Groux H, Bigler M, de Vries JE, et al., Inhibitory and
stimulatory effects of IL-10 on human CD8+ T cells. J Immunol 1998;
160(7): 3188.
- 37. Golab J, Interleukin 18--interferon gamma inducing
factor--a novel player in tumour immunotherapy? Cytokine 2000; 12(4):
332.
- 38. Nakanishi K, Yoshimoto T, Tsutsui H, et al.,
Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol
2001; 19(423.
- 39. Dinarello CA, Novick D, Puren AJ, et al., Overview of
interleukin-18: more than an interferon-gamma inducing factor. J Leukoc
Biol 1998; 63(6): 658.
- 40. Li Q, Carr AL, Donald EJ, et al., Synergistic effects
of IL-12 and IL-18 in skewing tumor-reactive T-cell responses towards a
type 1 pattern. Cancer Res 2005; 65(3): 1063.
- 41. Rodriguez-Galan MC, Bream JH, Farr A, et al.,
Synergistic effect of IL-2, IL-12, and IL-18 on thymocyte apoptosis and
Th1/Th2 cytokine expression. J Immunol 2005; 174(5): 2796.
- 42. Park HJ, Kim JE, Lee JY, et al., Increased expression
of IL-18 in cutaneous graft-versus-host disease. Immunol Lett 2004;
95(1): 57.
- 43. Scholl S, Sayer HG, Mugge LO, et al., Increase of
interleukin-18 serum levels after engraftment correlates with acute
graft-versus-host disease in allogeneic peripheral blood stem cell
transplantation. J Cancer Res Clin Oncol 2004; 130(12): 704.
- 44. Reddy P, Teshima T, Hildebrandt G, et al.,
Pretreatment of donors with interleukin-18 attenuates acute
graft-versus-host disease via STAT6 and preserves graft-versus-leukemia
effects. Blood 2003; 101(7): 2877.
- 45. Reddy P, Ferrara JL, Role of interleukin-18 in acute
graft-vs-host disease. J Lab Clin Med 2003; 141(6): 365.
|
|
LEGENDS
 |
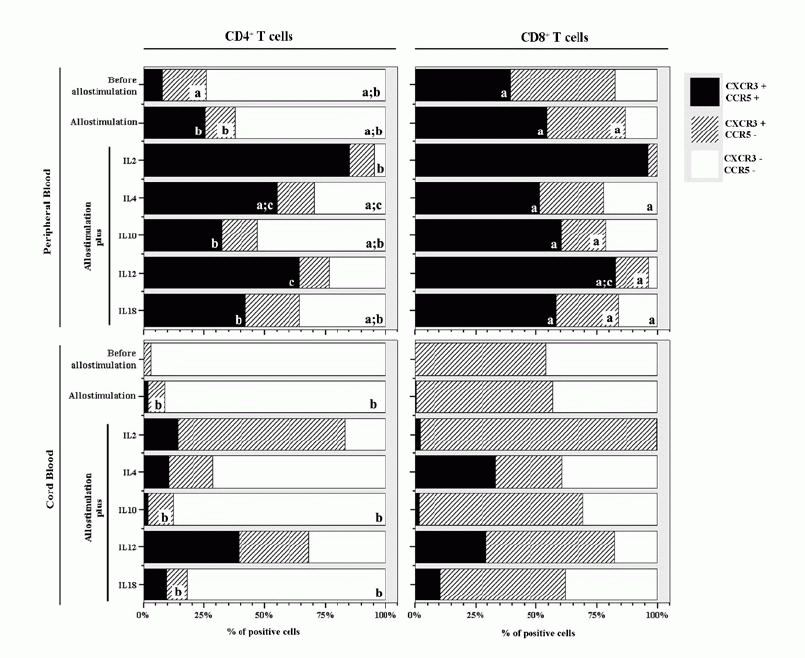
View larger version :
[in a new window] |
Figure 1- Expression of CXCR3 and CCR5 by CD8+ and CD4+
T cells from cord blood and adult peripheral blood before allogeneic
stimulation and after allogeneic stimulation in the absence or in the
presence of exogenous cytokines. Bars represent the median values of the
percentage of positive cells. Statistically significant differences were
considered when P<0.05. aP<0.05 between PB and CB; bP<0.05
between CD4+ and CD8+ T cells; cP<0.05
when compared to "Allostimulation without cytokine"?
|
![]() .
.
![]() . As expected, no CXCR3-/CCR5+ cells were found among
PBMCs or CBMCs.
. As expected, no CXCR3-/CCR5+ cells were found among
PBMCs or CBMCs.
![]()
![]() . However, in the CD4+ lymphocytes,the percentage of cells expressing
CCR5 is also enhanced in the presence of IL-4 [49(42-63)%] while the increases
that IL-10 and IL-18 induced on CCR5 expression CXCR3 [34(4,4-53)% and
45(5,7-54)% ] are considerably lower than that promoted by IL-2, IL-12 or IL-4
(Fig.1)
. However, in the CD4+ lymphocytes,the percentage of cells expressing
CCR5 is also enhanced in the presence of IL-4 [49(42-63)%] while the increases
that IL-10 and IL-18 induced on CCR5 expression CXCR3 [34(4,4-53)% and
45(5,7-54)% ] are considerably lower than that promoted by IL-2, IL-12 or IL-4
(Fig.1)![]() .
.
![]() .
.
![]() .
.
