Received- October 17, 2022; Accepted- November 10, 2022
International Journal of Biomedical Science
18(4), 60-65, Dec 15, 2022
© 2022 Yilin Chai. INTERNATIONAL ASSOCIATION OF BIOMEDICAL SCIENCES
Recent Advance in Understanding Vitamin D in Postpartum Depression
Yilin Chai
Barnard College, Columbia University, 3009 Broadway, New York, NY10027, USA
Corresponding Author: Yilin Chai, Barnard College, Columbia University, 3009 Broadway, New York, NY10027, USA. E-mail: yc4032@barnard.edu.
Running title: VITAMIN D AND POSTPARTUM DEPRESSION
|
|
ABSTRACT
 |
Postpartum Depression (PPD) is a prevalent mental disorder that affects approximately 20% women after giving birth worldwide. The common PPD symptoms include loss of appetite, suicidal thoughts, withdrawal from loved ones and babies. PPD not only affects mothers’ mental health but also results in mother-infant attachment and bonding problems. Though the exact PPD etiology remains poorly understood, cumulative studies have demonstrated that vitamin D deficiency during pregnancy is associated with PPD, and vitamin D3 supplementation during pregnancy greatly reduces PPD. This article specifically reviews the recent studies in the relationship between vitamin D3 deficiency and PPD, vitamin D3 treatment for PPD prevention, and the potential mechanisms by which vitamin D3 deficiency results in PPD.
KEY WORDS:
Vitamin D3; postpartum depression; vitamin D3 deficiency; vitamin D3 supplements; vitamin D3 mechanisms; 25-hydroxyvitamin D3; 1,25-dihydroxyvitamin D3
|
|
INTRODUCTION |
PPD is a type of depression in a woman that may begin in the first 4 weeks after giving birth, and it affects approximately 20% of women worldwide within the first month of parturition (1). The common PPD symptoms include suicidal thoughts or attempts, lack of energy, sense of emptiness and guilt, withdrawing from loved ones, feeling distant from babies, thinking about hurting themselves or their babies, etc (2). Different from “baby blues”, which typically begin within the first two to three days after delivery and only last for up to two weeks, PPD are more intense and last up to one year (3). As PPD can affect the health of women, the infants and families, and lead to chronic depressive disorder if not treated, an early diagnosis and timely treatments of PPD are essential (3).
Though its exact cause remains elusive, PPD etiology is believed to be associated with both genetics and environmental factors such as hormones, social, and psychological changes when having a baby (4). Vitamin D3 is a steroid hormone synthesized in the skin where 7-dehydrocholesterol is metabolized to Vitamin D3 in response to UVB light (5). Subsequently 25-hydorxylase in liver converts Vitamin D3 to 25-hydroxyvitamin D3 [25(OH)D3], which is then under hydroxylation reaction by specialized cells in the kidneys, brain and immune system, generating functional form of vitamin D3, 1,25(OH)D3, in the body (6). Dietary sources of vitamin D3 include fortified dairy or cereal products, fatty fish, eggs, and beef liver. Studies have shown that low vitamin D3 levels during pregnant women are associated with PPD and treatment vitamin D3 supplements during pregnancy helps decrease PPD (1). This review is aimed to summarize the studies related to the vitamin D3 deficiency as a risk factor for PPD, vitamin D3 supplementation for preventing PPD and the potential biological mechanisms of vitamin D3 in PPD.
|
|
ASSOCIATION BETWEEN VITAMIN D3 DEFICIENCY DURING PREGNANCY AND PDD |
Multiple studies have supported the inverse relationship between vitamin D3 deficiency during pregnancy and PPD. For instance, Robinson et al studied the serum 25(OH)D3 levels at 18-week gestation and risk of PPD in 796 Caucasian pregnant women in Perth, Australia (7). The women were grouped into 4 different quartiles based on their 25(OH)D3 concentrations (<19 ng/mL, <19 to 23 ng/mL, 24 to 28 ng/mL, and >28 ng/mL), and 25(OH)D3 levels from 20 to 50 ng/mL were considered normal in this study. All the women included in the study were followed for 3 days after baby delivery, and mood disturbances were measured using an index of 6 symptoms derived from the Edinburgh Postnatal Depression Scale (EPDS) including anxiety, sadness, mood fluctuation, appetite change, and sleep disturbance. They found that women with the lowest quartile of 25(OH)D3 levels (<19 ng/mL) at mid-pregnancy were more likely to report a higher level of PPD than women who were in the highest quartile of 25(OH)D3. They concluded that low vitamin D3 during pregnancy is a high-risk factor for the PPD development (7).
In consistency, Gur et al reported a negative correlation between levels of serum 25(OH)D3 in mid-pregnancy and the risk of PDD among a population of 208 pregnant women in Turkey. Serum 25(OH)D3 levels were groups as: severe deficiency (<10 ng/mL), mild deficiency (<20 ng/mL), and normal (>20 ng/mL). PPD was evaluated by the EPDS scoring system at three time points:1 week, 6 weeks and 6 months after baby delivery, respectively. They found that the mean 25(OH)D3 levels were significantly different between women with PPD and women without PPD in the first week, 6 weeks and 6 months after baby delivery. In contrast to only 11% of women in the control group with normal serum 25(OH)D3 experienced PPD at the sixth month postpartum, 50% of women in the severe 25(OH)D3 deficient group experienced PPD (P < 0.001) at the sixth month postpartum. The authors concluded that low levels of vitamin D3 during mid-pregnancy is closely associated with PPD (8). Similarly, Lamb et al conducted a study about the relationship between vitamin D3 deficiency and the risk of antenatal depression and PPD among pregnant women between 14 and 32 weeks of gestation in Southern California, USA. Depression was assessed at 14-week gestation, 32-week gestation, and 10-week postpartum by using the EPDS system (9). They observed an inverse relationship between vitamin D3 levels and EPDS scores at all time points including 10-week postpartum (9). Other studies that showed consistent results of vitamin D3 deficiency with PPD are listed in Table 1.
View this table:
[in a new window]
|
Table 1. Studies of the association of vitamin D deficiency with the risk of PPD.
| |
In contrast, Nielsen et al reported no correlation between vitamin D3 deficiency and the risk of PPD after comparing 605 women who filled antidepression medication prescriptions within 1 year after delivery and 875 women who did not fill any antidepressant prescription within 1 year of delivery. Both 25(OH)D3 and 25(OH)D2 were measured together from the sera that had been stored at –80°C for 9–15 years by the time of their study. They showed a J-shaped relationship between vitamin D3 concentrations and increased risks of PPD, suggesting that both high and low levels of vitamin D3 could be related to the risk of PPD. The discrepancy between Nielsen’s report and other studies of the inverse relationship of vitamin D3 and PPD could be attributed to several main reasons: 1) in Nielsen’s report, PPD is not determined by a reliable scoring tool such as EPDS, but instead by filling anti-depression medications which could result in enrollment mistakes as participants may experience other types of depression and/or other psychiatric conditions; 2) measuring total serum levels of both 25(OH)D3 and 25(OH)D2 while instead of only the bioactive serum 25(OH)D3 in Nielsen’s study; 3) The sera used for the Nielsen’s study were stored for 9-15 years, and such a long storage period may lead to degradation of some components in sera including vitamin D3 (10). In summary, cumulative research studies support that vitamin D3 deficiency during pregnancy is associated with PPD, highlighting the importance of vitamin D3 during pregnancy for PPD prevention.
|
|
VITAMIN D3 SUPPLEMENTATION DURING PREGNANCY FOR PPD PREVENTION |
As vitamin D3 deficiency is a high-risk factor for PPD, Vitamin D3 supplementation to treat PPD has been studied in recent years. For instance, Vaziri et al conducted a randomized clinical trial on pregnant women in Iran (16). 169 participants were randomly assigned into the placebo group and vitamin D3 supplementation group. The vitamin D3 supplementation group received 2000 IU of vitamin D3 daily from 26 to 28 weeks until childbirth. The EPDS was used to evaluate the depression scores at four time points during the study, namely at 26–28 and 38–40 weeks of gestation, and finally at 4 and 8 weeks after birth. No significant difference of depression scores of EPDS was observed between the placebo group and vitamin D3 group. While the vitamin D3 group had greater reduction in EPDS than the control group at 38–40 weeks of gestation (p=0.01), and at 4 and 8 weeks after birth (p<0.001) respectively, suggesting that vitamin D3 supplement is effective for decreasing PPD levels (16). In consistence, Amini et al reported the effectiveness of vitamin D3 supplementation in improving the symptoms of PPD. 81 participants with PPD EPDS scores under 12 were randomly assigned into three groups to receive either 50,000 IU vitamin D3 fortnightly + 500 mg calcium carbonate daily, or 50,000 IU vitamin D3 fortnightly + placebo of calcium carbonate daily, or placebo group for 8 weeks. Groups that received 50,000 IU vitamin D3 fortnightly with 500 mg calcium carbonate daily, and 50,000 IU vitamin D3 fortnightly with placebo of calcium carbonate daily had a greater reduction in PPD score than the placebo group. The result of this study supports that vitamin D3 supplement is effective in alleviating PPD symptoms (17). In consistency, Rouhi et al reported the similar conclusion on effectiveness of Vitamin D3 supplements on PPD. In this double-blind study, 80 primiparous women, who scored ≥13 on EPDS and ≥20 on the Fatigue Identification Form, were randomly assigned into two groups after giving birth. The control group received placebo pills daily and the vitamin D3 supplement group received 1000 IU daily for 6 months. They found that vitamin D3 supplements statistically significantly decreased depression EPDS scores and fatigue scores in the vitamin D3 treated group, suggesting that vitamin D3 supplements could be useful to reduce the PPD risk in women (18) (Table 2).
View this table:
[in a new window]
|
Table 2. Clinical trials of vitamin D supplements for PPD prevention and treatment.
| |
In addition, multiple studies have demonstrated that vitamin D3 supplementation improved the general depression conditions in women, and it is logical to reason that the effect of vitamin D3 supplementation on general depression in women shall be similar to PPD as PPD is a specific type of depression. Shipowick et al conducted a study on the relationship between vitamin D3 supplementation and depressive symptoms among women during the winter months (19). Participants are nine women with vitamin D3 level <40 ng/ml. In this study, Depression Inventory (BDI)-II was used to evaluate the depression severity. After 5,000 IU daily vitamin D3 supplementation for 8 weeks, the average of the BDI-II scores decreased by 10 points, suggesting that the vitamin D3 supplements can improve depression in women (19). Moreover, Khoraminya et al conducted a study to compare the therapeutic effects of vitamin D3 plus fluoxetine verse fluoxetine alone on major depression disorder (20). 42 participants with major depressive disorders were randomly assigned into two groups, receiving 1500 IU vitamin D3 plus 20mg fluoxetine or receiving fluoxetine alone for 8 weeks. They used Hamilton Depression Rating Scale (HDRA) as primary measurement for MDD and BDI as the secondary measurement for depressive outcome for 8 weeks. The HDRS and BDI of the group receiving 1500 IU vitamin D3 plus 20mg fluoxetine displayed more statistically significant decreases than those of the group receiving fluoxetine alone, suggesting that vitamin D3 is effective as a treatment to reduce depressive symptoms (20).
|
|
BIOLOGICAL BASIS AND POTENTIAL MECHANISMS OF VITAMIN D3 DEFICIENCY WITH PPD |
Though the pathological mechanism of vitamin D3 deficiency for PPD remains elusive, many studies have reported biological evidence that correlates vitamin D3 with depression. For example, [25(OH)D3] receptor (VDR) is widespread in brain areas that are involved in emotional processing and affective-related disorders associated with depression. Prufer et al reported that VDR is expressed in a variety of cells in the cerebellum, mesopontine area and diencephalon in rats (21). Similar findings were confirmed in the human brain. Eyles et al reported the VDR and 1α-hydroxylase (also known as CYP27B1) present in both neurons and glial cells in cortical regions, the prefrontal cortex, hypothalamus in humans (22). The widespread distribution of VDR in distinct portions of brain systems suggests an important function of vitamin D3 in the central nervous system.
Serotonin is a neurotransmitter that plays critical roles in the brain including appetite control, sleep, temperature, mood, and social cognition etc., and the low level of serotonin is believed to be associated with depression (23, 24). Evidence from “tryptophan depletion” studies demonstrated that recovered depressed patients can show clinically relevant depressive symptomatology, after lowering the serotonin activity through diminishing availability of tryptophan (25). All these studies suggest that impairing serotonin can be associated with clinical depression in some circumstances (25). Vitamin D3 has been demonstrated to regulate serotonin synthesis via the modulation of the tryptophan hydroxylase 2 gene expression in the brain, thus vitamin D3 deficiency could lead to low levels of serotonin in the brain, resulting in depression (26). Moreover, selective serotonin reuptake inhibitors, which increase the extracellular level of the neurotransmitter serotonin by limiting its reabsorption into presynaptic cells, have been widely used as anti-depression drugs (27).
In recent years, cumulative studies have supported that the immune system is implicated in the pathogenesis of depression. Individuals with depression often have higher levels of pro-inflammatory cytokine interleukin (IL)-1β and lower levels of anti-inflammatory cytokine IL-10 than non-depressed individuals (28, 29). Further studies have shown that medications used to treat depression decrease serum IL-1 β and interferon-y levels and stabilize the overproduction of inflammatory cytokines (30), indicating that inflammation is involved in depression. While vitamin D3 deficiency induces inflammatory responses in depressed people. For instance, Grudet et al found that low vitamin D3 may promote inflammation in depressed and suicidal subjects, and vitamin D3 was negatively correlated with inflammation in major depression disorder patients, but not in controls (31). Supplemental vitamin D3, along with calcium, has been found to decrease the biomarkers of inflammation (32, 33). Though no studies of inflammation of vitamin D3 in PPD, which is a subtype of depression, it is logical to assume that the inflammatory effects of vitamin D3 on general depression would be similar to PPD (Figure 1).
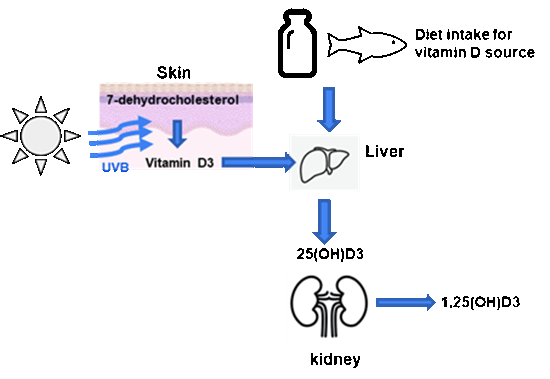
View larger version :
[in a new window] |
Figure 1. Metabolism of Vitamin D. 7-dehydrocholesterol in skin cells is metabolized to Vitamin D3 in response to UVB light, and Vitamin D3 is then converted to 25-hydroxyvitamin D3 (25(OH)D3) by 25-hydorxylase in liver converts. Subsequently 25(OH)D3 is hydroxylated to 1,25(OH)D3 mainly in the kidneys. In addition, vitamin D3 can also be obtained from dietary sources such as milk, fish, eggs, etc.
|
|
|
|
CONCLUSION |
Many studies have demonstrated that lower levels of vitamin D3 during pregnancy are associated with PPD in women, and vitamin D3 supplementation during pregnancy could reduce depressive symptoms. The exact mechanism by which vitamin D3 reduces PPD remains elusive. However, the widespread VDRs in brain areas associated with emotional processing and affective-related disorders offer the biological structural basis for vitamin D3’s function in PPD. Cumulative studies have indicated that vitamin D3 may exert its functions for depression by regulating the neurotransmitter serotonin and the pro-inflammatory cytokines. Nevertheless, further studies and larger scale clinical trials to solidify the relationship between vitamin D3 supplementation during pregnancy and PPD development is warranted.
|
|
CONFLICTING INTERESTS |
The author declared no potential conflicts of interest.
|
|
ABBREVIATIONS |
| [25(OH)D2] |
25-hydroxyvitamin D2 |
| [25(OH)D3] |
25-hydroxyvitamin D3 |
| BDI |
Depression Inventory |
| EPDS |
Edinburgh Postnatal Depression Scale |
| HDRA |
Hamilton Depression Rating Scale |
| MDD |
Major Depressive Disorder |
| PPD |
Postpartum Depression |
| VDR |
[25(OH)D3] receptor |
| 1,25(OH)D3 |
Vitamin D3 1,25-Dihydroxyvitamin D3 |
|
|
REFERENCES |
- Szpunar MJ. (2020). Association of antepartum vitamin D deficiency with postpartum depression: A clinical perspective. Public Health Nutrition. 2020; 23 (7): 1173–1178.
- Closa-Monasterolo R, Gispert-Llaurado M, Canals J, Luque V, et al. The Effect of Postpartum Depression and Current Mental Health Problems of the Mother on Child Behaviour at Eight Years. Maternal and Child Health Journa. 2017; 21 (7): 1563–1572.
- CDC. Depression Among Women. (Access date: July 23, 2022).
- Hasler G. PATHOPHYSIOLOGY OF DEPRESSION: DO WE HAVE ANY SOLID EVIDENCE OF INTEREST TO CLINICIANS? World Psychiatry. 2010; 9 (3): 155–161.
- Ellsworth-Bowers ER, Corwin EJ. Nutrition and the psychoneuroimmunology of postpartum depression. Nutrition Research Reviews. 2012; 25 (1): 180–192.
- Kesby JP, Eyles DW, Burne THJ, McGrath JJ. The effects of vitamin D on brain development and adult brain function. Molecular and Cellular Endocrinology. 2011; 347 (1–2): 121–127.
- Robinson M, Whitehouse AJO, Newnham JP, Gorman S, et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Archives of Women’s Mental Health. 2014; 17 (3): 213–219.
- Gur EB, Gokduman A, Turan GA, Tatar S, et al. Mid-pregnancy vitamin D levels and postpartum depression. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014; 179: 110–116.
- Lamb AR, Lutenbacher M, Wallston KA, Pepkowitz SH, et al. Vitamin D deficiency and depressive symptoms in the perinatal period. Archives of Women’s Mental Health. 2018; 21 (6): 745–755.
- Nielsen NO, Strøm M, Boyd HA, Andersen EW, et al. Vitamin D Status during Pregnancy and the Risk of Subsequent Postpartum Depression: A Case-Control Study. PLoS ONE. 2013; 8 (11): e80686.
- Accortt EE, Schetter CD, Peters RM, Cassidy-Bushrow AE. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Archives of Women’s Mental Health. 2016; 19 (2): 373–383.
- Brandenbarg J, Vrijkotte TGM, Goedhart G, van Eijsden M. Maternal Early-Pregnancy Vitamin D Status Is Associated With Maternal Depressive Symptoms in the Amsterdam Born Children and Their Development Cohort. Psychosomatic Medicine. 2012; 74 (7): 751–757.
- Fu CW, Liu JT, Tu WJ, Yang JQ, Cao Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG: An International Journal of Obstetrics & Gynaecology. 2015; 122 (12): 1688–1694.
- Murphy PK, Mueller M, Hulsey TC, Ebeling MD, Wagner CL. An Exploratory Study of Postpartum Depression and Vitamin D. Journal of the American Psychiatric Nurses Association. 2010; 16 (3): 170–177.
- Abedi P, Bovayri M, Fakhri A, Jahanfar S. The Relationship Between Vitamin D and Postpartum Depression in Reproductive-Aged Iranian Women. Journal of Medicine and Life. 2018; 11 (4): 286–292.
- Vaziri F, Nasiri S, Tavana Z, Dabbaghmanesh MH, et al. A randomized controlled trial of vitamin D supplementation on perinatal depression: In Iranian pregnant mothers. BMC Pregnancy and Childbirth. 2016; 16 (1): 239.
- Amini S, Amani R, Jafarirad S, Cheraghian B, et al. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: A randomized double-blind clinical trial. Nutritional Neuroscience. 2022; 25 (1): 22–32.
- Rouhi M, Rouhi N, Mohamadpour S, Tajrishi HPR. Vitamin D reduces postpartum depression and fatigue among Iranian women. British Journal of Midwifery. 2018; 26 (12): 787–793.
- Shipowick CD, Moore CB, Corbett C, Bindler R. Vitamin D and depressive symptoms in women during the winter: A pilot study. Applied Nursing Research. 2009; 22 (3): 221–225.
- Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Australian & New Zealand Journal of Psychiatry. 2013; 47 (3): 271–275.
- Prüfer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. Journal of Chemical Neuroanatomy. 1999; 16 (2): 135–145.
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. Journal of Chemical Neuroanatomy. 2005; 29 (1): 21–30.
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. The FASEB Journal. 2014; 28 (6): 2398–2413.
- Coppen A. The Biochemistry of Affective Disorders. British Journal of Psychiatry. 1967; 113 (504): 1237–1264.
- Smith, K., Fairburn, C., & Cowen, P. (1997). Relapse of depression after rapid depletion of tryptophan. The Lancet, 349(9056), 915–919.
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. The FASEB Journal. 2014; 28 (6): 2398–2413.
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. The FASEB Journal. 2014; 28 (6): 2398–2413.
- Lotrich FE. Inflammatory cytokine-associated depression. Brain Research. 2015; 1617: 113–125.
- Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between Pro- and Anti-inflammatory Cytokines, and between Th1 and Th2 Cytokines in Depressed Patients: The Effect of Electroacupuncture or Fluoxetine Treatment. Pharmacopsychiatry. 2009; 42 (05): 182–188.
- Hiles SA, Baker AL, de Malmanche T, Attia J. Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: A meta-analysis. Psychological Medicine. 2012; 42(10): 2015–2026.
- Grudet C, Wolkowitz OM, Mellon SH, Malm J, et al. Vitamin D and inflammation in major depressive disorder. Journal of Affective Disorders. 2020; 267: 33–41.
- Björkhem-Bergman L, Nylén H, Norlin AC, Lindh JD, et al. Serum Levels of 25-Hydroxyvitamin D and the CYP3A Biomarker 4 β -Hydroxycholesterol in a High-Dose Vitamin D Supplementation Study. Drug Metabolism and Disposition. 2013; 41 (4): 704–708.
- Hopkins MH, Owen J, Ahearn T, Fedirko V, et al. Effects of Supplemental Vitamin D and Calcium on Biomarkers of Inflammation in Colorectal Adenoma Patients: A Randomized, Controlled Clinical Trial. Cancer Prevention Research. 2011; 4 (10): 1645–1654.