International Journal of Biomedical Science
2(1), 64-72, Feb 15, 2006
© 2005 Master Publishing Group
Suppressive Effects of Intrathecal Application of Diazepam
on Visceral Pain and Hyperalgesia Induced
by Intracolonic Instillation of Formalin
Jinghui Huanga, Libing Liub*,
Yumei Zhoub, Jun Yub,
Jiao Denga
a Cadet Brigade Squadron Twelve, Fourth Military Medical University, Xi’an 710032, China
b Center of Teaching Lab, School of Basic Medicine, Fourth Military Medical University, Xi’an 710032, China
*Corresponding author: Address: Center of Teaching Lab, School of Basic Medicine, Fourth Military Medical University, No. 17 Changle Xi Lu, Xi’an, 710032, Shaanxi, Peoples Republic of China; Tel: 86-29-84774764; Fax: 86-29-84773159
Email: liulibing@fmmu.edu.cn
|
|
ABSTRACT
 |
Using an animal model of colonic inflammation, the effects of intrathecal (i.t.) diazepam (0.02mg/kg, 0.08mg/kg and 0.15mg/kg) on visceral pain-related behaviors and hyperalgesia associated with colonic inflammation were investigated. In this visceral pain model, acute visceral pain response was induced by intracolonic (i.c.) injection of 0.5 ml of dilute formalin (2%, 5% and 10%) in rats, and the peak pain behavioral response and hyperalgesia were evoked by i.c. 5% formalin. I.t. diazepam (0.02mg/kg, 0.08mg/kg and 0.15mg/kg) followed 10 min later by i.c. injection of 5% formalin, attenuated the visceral pain behaviors induced by 5% formalin in a dose dependent manner. Of the three doses tested, the duration of the suppressive effect of 0.15mg/kg diazepam on visceral pain was the longest, which is 60 min compared with 45 min at other two doses. Moreover, i.t. pretreatment with 0.08mg/kg diazepam attenuated the hyperalgesia induced by i.c. injection of 5% formalin. The findings in our studies shown that i.t. diazepam had a suppressive effect on visceral pain associated with noxious stimulation of colon, and provided evidence that diazepam may be used as an analgesic drug in the future.
KEY WORDS:
Colonic inflammation; Diazepam; Formalin; Hyperalgesia; Visceral pain
|
|
INTRODUCTION |
Visceral pain is the most common form of pain produced by disease,
and one of the most frequent reasons why patients seek medical attention.
Nonetheless, most of what we know about pain mechanisms is derived from studies
of somatic rather than visceral nociception. However, the more we know about the
mechanisms of somatic and visceral sensation, the more we realize that these two
processes, while having many common features, also have important differences.
For example, visceral pain is characterized by its referral to the body wall. In
the zone of referral, patients also report tenderness known as referred visceral
hyperalgesia.9
γ-Aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in
virtually every area of the central nervous system (CNS), and it activates GABAA
receptors to open chloride ion channels and mediates fast inhibitory synaptic
transmission. GABAA receptors belong to the family of homologous pentameric
transmitter-gated ion channels and many classes of drugs interact with them.11
Among these drugs are the positive allosteric modulators acting at the
benzodiazepine binding site, which is an integral component of this pentameric
chloride-selective pore complex.20 Diazepam, a benzodiazepine receptor agonist,
has been shown to act as a positive allosteric modulator to enhance GABA-mediated
Cl- conductance in cultured/dissociated central neurons and brain tissue
slices.2, 3, 19 Potentiation of GABAA receptor mediated synaptic inhibition is
believed to contribute to the anxiolytic, anticonvulsant, and sedative effects
of diazepam.23
The rat sacral dorsal commissural nucleus (SDCN), which represents the dorsal
gray matter of the central canal in the lower lumbar and sacral spinal cord,
serves as a relay of sensory information from the pelvic viscera. Moreover,
previous studies have revealed that GABA-like immunoreactive neurons and
terminals and GABA receptors are densely located in the SDCN.5 All these
evidences indicated that modulation of GABAA receptors by benzodiazepines has
the potential for significant influence on spinal nociception. In addition,
there are reports that intrathecal (i.t.) diazepam suppresses somatic sensation
in pentobarbital-anesthetized rats.18 However, little information about the
suppressive effect of diazepam on visceral pain was reported until now. So, in
this study, on the basis of a visceral-specific behavioral model developed by
intracolonic (i.c.) instillation of formalin, the possible effects of diazepam
on visceral pain-related behaviors and hyperalgesia associated with colonic
inflammation were investigated. It is hoped that information obtained will lead
to clinical implications that diazepam have a suppressive effect on visceral
pain when it’s used as anxiolytics and anticonvulsants.
|
|
MATERIALS AND METHODS
 |
Animals
Adult male Sprague-Dawley albino rats weighing from 180 to 220 g were provided
by Laboratory Animal Center of the Fourth Military Medical University (FMMU) and
use of the animals was reviewed and approved by the Institutional Ethical
Committee of the FMMU. The IASP's guidelines for pain research in animals were
followed. The animals were housed in plastic boxes in group of 3 with food and
water available ad libitum in a colony room with controlled temperature (24 2
℃), humidity (50% ~ 60%), and a 12:12 h light-dark cycle. The rats were
acclimatized to the laboratory and habituated to the observation chamber for at
least 30 min each day for 5 days before testing.
Spinal catheterization procedure
For i.t. administration of the drugs, chronic i.t. catheterization was modified
according to previous reports.22 Briefly, a PE-8 plastic tube (inter
diameter=0.28 mm; out diameter=0.60 mm, Natume, Tokyo, Japan) was inserted from
the T2–T3 level into the subarachnoid space of the rostral lumbosacral
enlargement under continued intraperitoneal pentobarbital sodium anesthesia (40
mg/kg). Experiments were performed at least 5 days after the operation to
minimize any influence of the catheterization procedure. Only those without
motor disturbances or other abnormal signs were used. After tests, each rat was
checked for the placement of the end of the catheter, those with incorrect
placement or local pathological changes were not used for experimentation.
Nociceptive behavior assessment
A 30 cm×30 cm×30 cm transparent observation chamber with a transparent glass
floor was placed on a supporting frame of 45 cm high above the experimental
table. Before starting the session, the rat was placed in the observation
chamber for 30 min to accommodate to its new environment. Petroleum jelly
(Vaseline) was then applied in the perianal area to avoid the stimulation of
somatic areas by contact with the irritant chemicals. Then the animal was
anesthetized with a small amount of diethyl ether, while 0.5 ml of solution
(saline, 2% formalin, 5% formalin or 10% formalin) was administered by
introducing a fine cannula with a rounded tip (inter diameter=1.1 mm; out
diameter=1.5 mm, 8 cm long) into the colon via the anus. The rat was replaced in
its observation chamber. The spontaneous behaviors were observed for 90 min, and
the latency of the first such behavior was also noted. All selected behaviors
related to visceral nociception (i.e., licking or nibbling abdomen or perineal
area ( ), body stretching ( ), contraction of the flanks ( ), and whole body
contraction ( )), were listed here in increasing order of nociceptive intensity.
The latter behavior ( ) was further graded according to the duration of the
given episode: for less than 30 s, between 30 s and 1 min, and for more than 1
min. The nociceptive response ( ) was then calculated for each of the successive
15 min periods, using the formula: .17 In all experiments, animal behaviors were
recorded blind as regarded control or treated status.
Examination of mechanical hyperalgesia
In the rats with i.c. saline and 5% formalin, the frequencies of withdrawal
responses to von Frey hairs of the abdomen, the hind paws and the tails were
examined as tests of referred hyperalgesia immediately after behavioral tests.
Five hairs with forces of 47, 60, 80, 115, 156 mN were applied 10 times each in
ascending order of force, and the number and intensity of responses noted. The
forces exerted by the von Frey hairs were checked before testing using a
micromanipulator to advance them towards a sensitive balance. The hair was
applied for 1~2 s, with an inter-stimulus interval of 5~10 s. Care was taken not
to stimulate the same point twice in succession, to avoid ‘wind-up’ effects or
desensitization. The appearance of a sharp withdrawal of the stimulated region
on the application of a hair was considered as a withdrawal response. In
addition, immediate licking or scratching the stimulated site and jumping were
also regarded as withdrawal responses for abdomen.12
Assessment of colonic inflammation
At the end of behavioral test, one rat with i.c. saline and one with i.c. 5%
formalin were chosen randomly, and they were killed by an intraperitoneal
pentobarbital overdose. The colon was excised and opened longitudinally.
Histological assessment was then performed. The tissue was removed, fixed
overnight by immersion in Bouin’s solution, dehydrated, embedded in paraffin and
stained with haematoxylin and eosin.
Effects of i.t. diazepam on visceral pain-related behaviors and hyperalgesia
induced by i.c. 5% formalin
Four groups of rats (n=8 per group) were intrathecally pre-treated with 10 µL of
diazepam at three doses (0.02mg/kg, 0.08mg/kg and 0.15mg/kg) and with saline
vehicle 10 min before i.c. 5% formalin to examine the effects of i.t. diazepam
on visceral pain–related behaviors.
In the rats intrathecally pretreated with 10 µL of saline and diazepam
(0.08mg/kg), the responses to mechanical stimulation of the abdomen, the tail
and the hind paws were tested with von Frey hairs as described above.
Statistical analysis
The SPSS software was used to determine the statistical significance of the
differences. The results were expressed as means ± S.E.M. of the value of pain
scored per 15 min, of the latency of the first visceral pain behavior and of the
response frequency at each von Frey hair. Group differences were considered
statistically significant at P<0.05 using a repeated-measures analysis of
variance for visceral pain-related behaviors recorded along, test for the
response frequency at each intensity of von Frey hair, and test for the latency
of the first visceral pain behavior.
 |
RESULTS |
Formalin induced inflammation in the colon
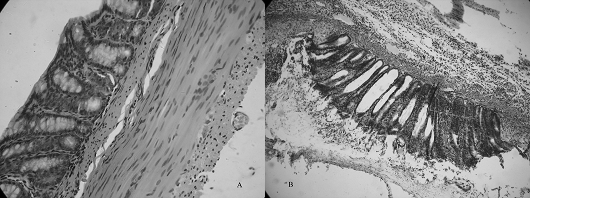
Gross inspection of the colon after i.c. saline showed an apparently normal
colon as shown in Fig.1A. However, I.c. 5% formalin evoked plasma extravasation
in the colon that was significantly different from that observed after saline
administration. As shown in Fig.1B, The architecture of the mucosa was
disorganized and the loss of crypt epithelial cells can be seen. Mild cellular
infiltration in lamina propria is also noted.
View larger version :
[in a new window] |
Fig. 1 Colonic inflammation induced by i.c. 5% formalin.
| |
Nociceptive responses after i.c. injection of
formalin and saline
I.c. saline was followed by the appearance of a small number of abdominal
licking behaviors, while i.c. formalin produced a tonic, mono-phasic nociceptive
response lasted for about 75 min, which is significantly different from that
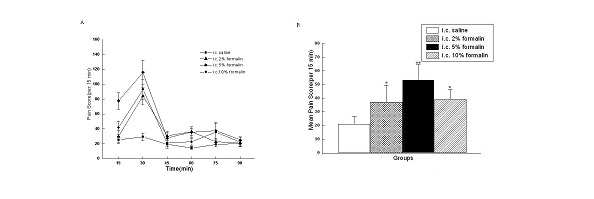
observed in saline-treated rats (Fig.2). The number of behaviors evoked by
formalin was concentration-dependent, with more behavioral responses evoked by
higher concentrations, but 5% formalin evoked more visceral pain-related
behaviors than 2% and 10% formalin (Fig.2B). Moreover, there was also evidence
of a ‘ceiling effect’ with the highest concentration tested. During the 90 min
test period, there was a maximal visceral pain response at 15 min-30 min at all
concentrations tested, then the number of visceral pain-related behaviors
decreased to baseline level at 75 min after formalin injection.
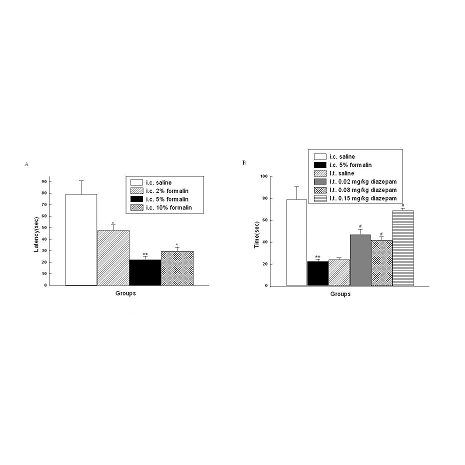
The latency of the response to administration of saline was greater than 40 s in
all rats tested. In contrast, the latency was much shorter when i.c.
administrated of formalin. As shown in Fig.3A, the latency of the response to
administration of saline, 2% formalin, 5% formalin, 10% formalin were 79.1±11.8
s, 47.8±4.8 s, 22.5±1.4 s, 29.8±2.5 s.
View larger version :
[in a new window] |
Fig. 2 Visceral pain responses induced by formalin. (A) Curve graph shows the mean time courses for 90 min after i.c. formalin. (B) Column graph shows the mean numbers of the pain scores per 15 min averaged from 6 time blocks of 90 min after i.c. formalin. *P<0.01; **P<0.001vs rats with i.c. saline.
| |
View larger version :
[in a new window] |
Fig. 3 Latency of the first visceral pain-related behavior. *P<0.05, **P<0.01 vs rats with i.c. saline. #P<0.05 vs rats with i.c. 5% formalin.
| |
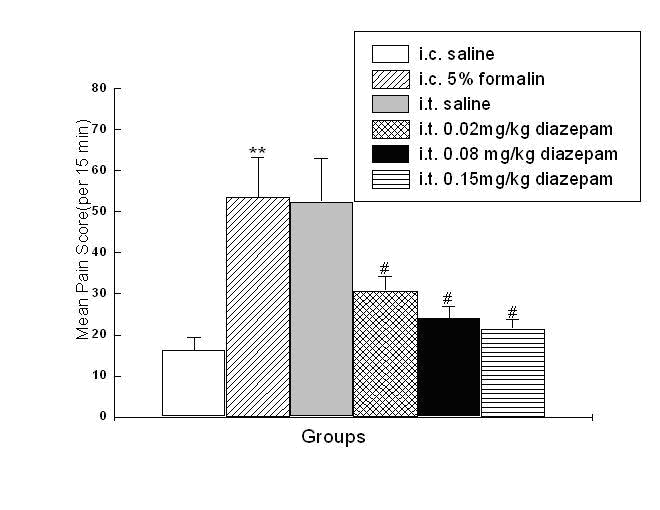
Effect of i.t. diazepam on spontaneous behaviors
evoked by i.c. 5% formalin
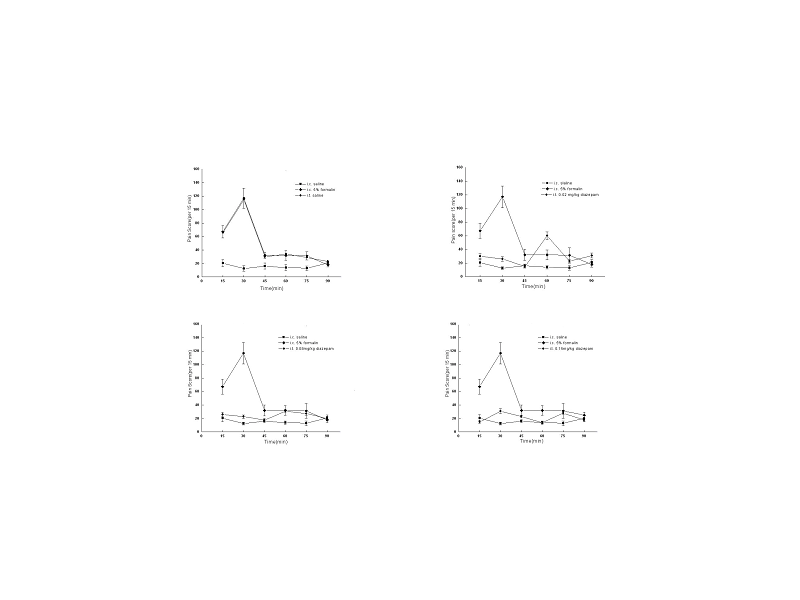
I.t. saline had little influence on visceral pain-related behaviors induced by
5% formalin, while i.t. diazepam dose-dependently reduced the number of visceral
pain-related behaviors induced by i.c. 5% formalin treatment (Fig.5), with
higher dose intrathecally administrated, to more extent the number of behaviors
was reduced. As shown in Fig. 4, the duration of the suppressive effect of i.t.
diazepam on visceral pain behaviors lasted for 45 min-60 min after i.c.
administration during the 90 min test period, which was also dose dependent,
with 60 min at 0.15mg/kg, and 45 min at other two doses (0.02mg/kg, 0.08mg/kg).
The latency of the response to i.c. 5% formalin treatment in the rats with i.t.
diazepam was much longer than that observed in the rats without i.t. diazepam.
As shown in Fig.3B, the latency of the response to i.c. 5% formalin in the rats
with i.t. saline and i.t. diazepam (0.02mg/kg, 0.08mg/kg and 0.15mg/kg) were
24.1 ± 1.7 s, 47.3 ± 5.3 s, 42.3 ± 3.5 s, 69.2 ± 7.2 s, respectively.
View larger version :
[in a new window] |
Fig. 4 Effect of i.t. diazepam on visceral pain-related behaviors induced by i.c. 5% formalin.
| |
View larger version :
[in a new window] |
Fig. 5 Effect of i.t. diazepam on visceral pain-related behaviors induced by i.c. 5% formalin. **P<0.001 vs rats with i.c. saline; #P<0.001 vs rats with i.c. 5% formalin.
| |
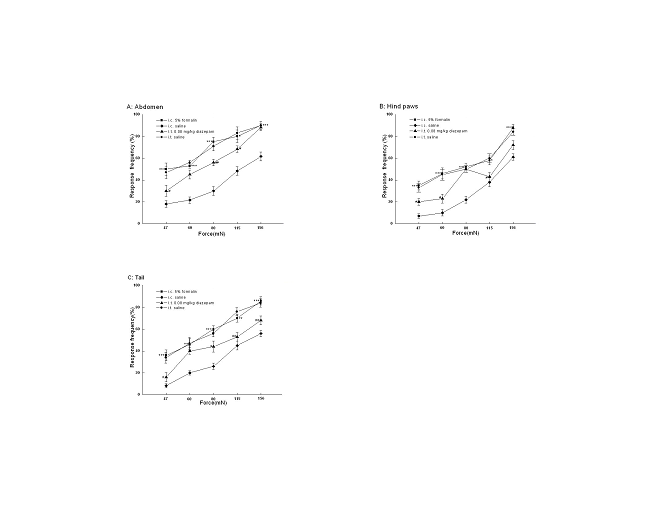
Withdrawal responses to
mechanical stimulations
The tail, the hind paws and the abdomen were similar sensitive when tested their
responsiveness to von Frey hairs 90 min after i.c. saline. However, 90 min after
i.c. 5% formalin treatment, there was a marked increase in responsiveness in all
three sites tested, such that the stimulus response curve shifted to the left
(Fig. 6), which was called hyperalgesia. This phenomenon was attenuated by i.t.
diazepam to some extent. As shown in Fig. 6, Rats with i.t. diazepam (0.08mg/kg)
were less responsive to von Frey hairs than the ones without i.t. diazepam 90
min post i.c. 5% formalin treatment, with a pronounced shift of
stimulus-response curve to the right (Fig.6).
View larger version :
[in a new window] |
Fig. 6 Responses to mechanical stimulation of different body areas with von Frey hairs of five intensities. *P<0.05; **P<0.01, ***P<0.001 vs rats with i.c. saline. #P<0.05, ##P<0.01vs rats with i.c. 5% formalin.
| |
 DISCUSSIONS DISCUSSIONS

|
Abdominal licking has been described as a common response in practically all
models of cystitis, ureteric calculosis, uterine inflammation and stimulation of
colon.8,10,17,26 However, abdominal reaction (body stretching, contraction of
the flanks and whole body contraction) seems to be more specifically associated
with noxious stimulation of colon in both rats and mice,17 although it has also
been reported in some studies of cystitis in rats.10 So, abdominal licking and
abdominal reaction were chosen as indexes of visceral pain in this visceral pain
model, and in our studies, i.c. formalin produced a tonic, mono-phasic
nociceptive response which is different from biphasic nociceptive response
induced by colonic wall injection of formalin.17 Moreover, ‘ceiling effect’
noted with the higher concentration of formalin tested in our experiment, may be
a behavioral phenomenon in which the active expression of pain-related behaviors
are partially occluded by freezing reaction. Alternatively, it may be due to a
local desensitization resulting from mucosal damage, as has been described for
relax responses to colon distension after mucosal inflammation.6
Benzodiazepines exert their effect by binding to a specific site in the
macromolecular complex of the GABAA receptor, increasing their affinity for GABA.
This results in an increased opening frequency of these ligand-gated Cl-
channels, thus potentiating the effect of GABA release in terms of inhibitory
effects on the postsynaptic cells.21 Several studies indicated i.t. diazepam
suppressed nociceptive reflexes in pentobarbital-anesthetized rats and the
nociceptive activity of convergent neurons in the spinal cord during ischemia
and reperfusion of their receptive fields on the rat's tail, both of which were
related with somatic sensation.7,18 Our present results confirmed the
antinociceptive effects of diazepam in terms of visceral sensation using a model
of inflammatory colonic pain. However, the exact mechanisms of the inhibitory
effect of i.t. diazepam on visceral pain were still unclear, but recent
evidences suggest that the interaction between diazepam and GABAA receptor
complex in the SDCN might be one of the underlying mechanisms.
SDCN is a cell column located in the dorsal gray matter of central canal in the
lower lumbar and sacral spinal cord.13 It receives primary afferent inputs from
both pelvic and pudendal nerves, which provide major innervation of pelvic
organ.14, 24 Accordingly, the SDCN serves as a relay of sensory information from
the pelvic viscera. An important role of the nucleus is in nociception, as
suggested by the increased expression of c-fos in the SDCN following both
noxious chemical stimulation of the bladder and high-intensity electrical
stimulation of the pelvic nerve.4, 13 The opinion that the projections from the
SDCN participate in nociception was also indicated by clinical evidence, that
discrete lesions of the dorsal columns relieved pelvic pain in patients.28 Axons
of neurons in SDCN have been shown to travel in the dorsal columns to the
gracile nucleus and in the ventrolateral quadrant to reticular formation.1, 25
Furthermore, neurons in this region receive descending inputs from several
regions of brain known to modulate the spinal processing of nociceptive
signals.15, 27 These characteristics thus make the SDCN an ideal place to study
the transmission and modulation of the pelvic visceral nociceptive signals.
Moreover, previous studies have revealed that GABA-like immunoreactive neurons
and terminals and GABA receptors are densely located in the SDCN.3, 5 In our
studies, diazepam was injected into the level of lower lumbar and sacral spinal
cord, and the diazepam might exert its suppressive effect on visceral pain by
potentiating the inhibitory effects of GABA through facilitating the uptake of
Cl- into GABAergic neurons in SDCN, and it might be possible that in such a way
the transmission of visceral pain information form periphery to the higher
centers of the brain was partly inhibited. However, the effect of diazepam in
the SDCN might be just one possible reason count for its suppressive effect on
visceral pain. To discover the exact mechanisms of the analgesic action of i.t.
diazepam, more works need to be done in the future.
Visceral pain differs from somatic pain in several clinical and physiological
aspects and in particular, pain is often perceived by the sufferer as arising
from somatic sites distant from the visceral injury.16 This phenomenon was
called visceral-somatic hyperalgesia, which has been qualified using electrical
and nature stimulation in patients with a variety of different visceral pain
states, and has also been measured in animal models of visceral pain. It has
been documented that in rats with an experimental ureteric calculosis a reduced
threshold to electrical stimulation of the flank muscles was observed.10 What’s
more, rats with uterine inflammation show increased responsiveness to palpation
of the lateral flank muscles.26 In our studies, rats with colonic inflammation
are more sensitive to mechanical stimulation in the abdomen, the hind paws and
the tail. These sites are commonly innervated by dermatomes which share the same
spinal innervation as the injured viscera, which has been previously reported in
an animal model of mouse colonic inflammation.12 our studies shown that the
referred punctuate mechanical hyperalgesia was attenuated by i.t. diazepam. This
provides supporting evidence for the possibility that diazepam may be effective
in preventing pain associated with inflammatory conditions of the colon. These
findings may be of particular clinical importance since, in chronic visceral
hyperalgesia syndromes, referred hyperalgesia can be a cause of suffering long
after the immediate consequences of the primary insult has been resolved.11
In conclusion, the results have shown that intrathecal diazepam is effective in
attenuating visceral pain-related behaviors and referred hyperalgesia in an
animal model of colonic inflammation. However, there are a lot of problems to
solve before we use diazepam as an intrathecal drug to relieve visceral pain in
clinical settings. When diazepam is used intrathecally in humans, whether there
are adverse or side effects? Are there any other therapeutic functions? And what
is the appropriate dose to relieve visceral pain. To answer all these questions,
further studies are needed to be carried out.
|
|
REFERENCES
 |
- 1.
Al-Chaer ED, Lawand NB, Westlund KN, et al.
Pelvic visceral input into the nucleus gracilis is largely mediated by the
postsynaptic dorsal column pathway. J Neurophysiol 1996; 76: 2675–2690.
-
2.
Alsbo CW, Kristiansen U, Moller F, et al. GABAA
receptor subunit interactions important for benzodiazepine and zinc modulation:
a patch-clamp and single cell RT-PCR study. Eur J Neurosci 2001; 13: 1673–1682.
-
3.
Bickford PC, Breiderick L. Benzodiazepine
modulation of GABAergic responses is intact in the cerebellum of aged F344 rats.
Neurosci Lett 2000; 291: 187–190.
-
4.
Birder LA, Roppolo JR, Iadarola MJ, et al.
Electrical stimulation of visceral afferent pathways in the pelvic nerve
increases c-fos in the rat lumbosacral spinal cord. Neurosci Lett 1991; 129:
193–196.
-
5.
Bohlhalter S, Weinmann O, Mohler H, et al. Laminar
compartmentalization of GABAA receptor subtypes in the spinal cord: an
immunohistochemical study. J Neurosci 1996; 16: 283–297.
-
6.
Burton MB, Gebhart GF. Effects of intracolonic
acetic acid on responses to colorectal distension in the rat. Brain Res 1995;
672: 77-82.
-
7.
Cartmell SM, Mitchell D. Diazepam attenuates
hyperexcitability and mechanical hypersensitivity of dorsal horn convergent
neurones during reperfusion of the rat's tail following ischaemia. Brain Res
1994; 659: 82 – 90.
-
8.
Craft RM, Carlisi VJ, Mattia A, et al. Behavioral
characterization of the excitatory and desensitizing effects of intravesical
capsaicin and resiniferatoxin in the rat. Pain 1993; 55: 205-215.
-
9.
Giamberardino MA. Recent and forgotten aspects of
visceral pain. Eur J Pain 1999; 3: 77-92.
- 10.
Giamberardino MA, Valente R, de Bigontina P, et al.
Artificial ureteral calculosis in rats: behavioural characterization of visceral
pain episodes and their relationship with referred lumbar muscle hyperalgesia.
Pain 1995; 61: 459-469.
- 11.
Johnston GAR. GABAA receptor pharmacology.
Pharmacol Ther 1996; 69: 173–198.
-
12.
Laird JM, Martinez-Caro L, Garcia-Nicas E, et al. A
new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001;
92: 335-342.
- 13.
Lu Y, Jin SX, Xu TL, et al. Expression of c-fos
protein in substance P receptor-like immunoreactive neurons in response to
noxious stimuli in the urinary bladder: an observation in the lumbosacral cord
segments of the rat. Neurosci Lett 1995; 198: 139–142.
-
14.
Lu Y, Li JS, Qin BZ. Morphological evidence that dorsal commissural nucleus of
rat lumbosacral spinal cord receives noxious information from the pelvic
viscera. Chin J Neuroanat 1996; 13: 214 –223.
- 15.
Martin GF, Cabana T, Ditirro FJ, et al. Raphespinal
projections in the North American opossum: evidence for connectional
heterogeneity. J Comp Neurol 1982; 208: 67–84.
-
16.
McMahon SB. Are there fundamental differences in the peripheral mechanisms of
visceral and somatic pain? Behav Brain Sci 1997; 20: 381-391.
- 17.
Miampamba M, Chery-Croze S, Detolle-Sarbach S, et
al. Antinociceptive effects of oral colidine and S12813-4 in acute colon
inflammation in rats. Eur J Pharmacol 1996; 308: 251 -259.
-
18.
Pomeranz B, Nguyen P. I.t. diazepam suppresses
nociceptive reflexes and potentiates electroacupuncture effects in
pentobarbital-anesthetized rats. Neurosci Lett 1987; 77: 316-320.
-
19.
Poncer JC, Durr R, Gahwiler BH, et al. Modulation
of synaptic GABAA receptor function by benzodiazepines in area CA3 of rat
hippocampal slice cultures. Neuropharmacology 1996; 35: 1169–1179.
-
20.
Sigel E, Buhr A. The benzodiazepine binding site of
GABAA receptors. Trends Pharmacol Sci 1997; 18: 425–429.
-
21.
Wikinski SI, Acosta GB, Gravielle MC, et al.
Diazepam fails to potentiate GABA-induced chloride uptake and to produce
anxiolytic-like action in aged rats. Pharmacol Biochem Behav 2001; 68: 721-727.
- 22.Storkson
RV, Kjorsvik A, Tjolsen A, et al. Lumbar catheterization of the spinal
subarachnoid space in the rat. J Neurosci Methods 1996; 65: 167–172.
-
23. Tanelian DL, Kosek
P, Mody I, et al. The role of the GABAA receptor/chloride channel complex in
anesthesia. Anesthesiology 1993; 78: 757–776.
-
24. Thor KB, Morgan C,
Nadelhaft I, et al. Organization of afferent and efferent pathways in the
pudendal nerve of female cat. J Comp Neurol 1989; 288:263–279.
-
25.
Wang CC, Willis WD, Westlund KN. Ascending
projections from the area around the spinal cord central canal: a Phaseolus
vulgaris leucoagglutinin study in rats. J Comp Neurol 1999; 415: 341–367.
-
26.
Wesselmann U, Czakanski PP, Affaitati G, et
al. Uterine infammation as a noxious visceral stimulus: behavioral
characterization in the rat. Neurosci Lett 1998; 246: 73-76.
-
27.
Westlund KN, Coulter JD. Descending projections of
the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey:
axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry.
Brain Res 1980; 2: 235–264.
-
28.
Willis WD, Al-Chaer ED, Quast MJ, et al. A visceral
pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci USA
1999; 96: 7675–7679.