| Original Article |
|
|
1. Department of Nephrology, Third Xiangya Hospital, Central
South University, Changsha 410013, China
2. Department of Nephrology, Second Xiangya Hospital, Central South University,
Changsha 410011, China
|
|
ABSTRACT |
|
|
INTRODUCTION |
|
|
MATERIALS AND METHOLD |
|
|
RESULTS |
|
|
DISCUSSIONS |
|
|
REFERENCES |
|
|
ABSTRACT
|
|---|
KEY WORDS: mesothelial cells; TGF-β1; Smad2/3; Smad7;
extracellular matrix
|
|
INTRODUCTION
|
|---|
Ultrafiltration failure caused by peritoneal fibrosis is the major cause for patients with continuous ambulatory peritoneal dialysis (CAPD) to give up peritoneal dialysis therapy. The increase in endogenous TGF-β1 induced by a variety of intrinsic or extrinsic factors results in excessive accumulation of extracellular matrix around HPMCs, which contributes to the formation of peritoneal fibrosis[1,2]. Smad signaling pathway was found recently to be an important TGF-β1 downstream signaling pathway, which was closely related to fibrosis in many tissues such as cardiac muscle, liver, lung and kidney [3,4]. Does TGF-β1 dependent Smad signaling pathway exert its effect in the process of peritoneal fibrosis? However, no definite answers are available as yet. Therefore, it is necessary for us to elucidate the underlying mechanisms for the development of peritoneal fibrosis, and further to find safe and effective methods to elongate dialysis time and improve life quality of patients with peritoneal dialysis.
|
|
MATERIALS AND METHODS
|
|---|
Reagents
Goat anti-human phosphorylated Smad2/3 ( P-Smad2/3 ) polyclonal antibody and
goat anti-human Smad7 polyclonal antibody were purchased from Santa Cruz
Company, USA; mouse anti-human CTGF(connective tissue growth factor) monoclonal
antibody was purchased from R&D Company, USA; mouse anti-humanα-SMA monoclonal
antibody, mouse anti-human COL1 (human collagen type 1) and pure TGF-β1 were
purchased from Sigma Company,USA. ELISA kits for Plasminogen Activator Inhibitor
type 1 (PAI-1) and fibronectin (FN) were purchased from Shanghai Sun
Biotechnology Company. Immunohistochemistry staining kits were purchased from
Beijing Zhongshan Biotechnology Co. Ltd.
Isolation, culture and identification of
HPMCs [5]
Dissection of greater omentum from healthy male adults with removal of blood
vessel and lipid tissue was performed in sterile room where tissue was dissected
into 1×1cm2 pieces, washed twice with Phosphate Buffered Saline (PBS)
and F12 respectively. After the tissue pieces were transferred into sterile
centrifuge tube containing 20ml 0.125% pancreatin and 0.01% EDTA , and the
cultured cells were incubated on rocking bed at 250 rpm at 37oC for
15min. The digestion was stopped by addition of 3ml 15% Fetal Calf Serum
(FCS)-F12 and the samples centrifugated at 1000rpm at 40C for 10min. The pellet
was blown once with 5ml F12, centrifugated at 1000rpm at 4oC for 5min
and after removal of supernatant, 5ml of complete medium ( 15%FCS-F12, 0.5μg/ml
insulin, 1μg/ml hydrocortisone, 5μg/ml transferring, 2μmol/ml glutamine) was
added to the cells. The cell suspension was transferred into 25cm2
culture flask coated with 0.1% gelatin and containing culture media? And
supplemented with 100U/ml penicillin and 100U/ml streptomycin. The culture
flasks were placed into incubator at 37oC, 5% CO2 for cell culturing.
Cell culture media was refreshed every three days. When the cultured cells were
grown into confluence, the cells were detached with 0.125% pancreatin-0.01% EDTA
and plated. The third passage of the cells was used in experiments. 95% of the
cells of the third passage were found to posses' characteristics of mesothelial
cells by inverted microscopy, transmission electron microscopy and scanning
electron microscopy. Cytokeratin and VIII factor immunohistochemistry staining.
Immunohistochemistry analysis of
intracellular Smad2/3 phosphoralation
The confluent cells of the second passage were adjusted to the concentration of
1×105/ml, and plated on cover glass, which was placed in 24-well culture plate.
After cells reached confluence, 0.1% FCS-F12 was added, synchronized for 24h,
and after they entered the resting stage the cells were divided into 0, 15min,
30min, 1h, 2h and incubated with 5ng/ml TGF-β1. After specified periods of time
the supernatant was removed and the cover glass was taken out and fixed with
pure acetone. SP method was used in p-Smad2/3 immmohistochemicalstaining
(1:100). In control samples PBS was added instead of primary antibody. The
translocation of p-Smad2/3 from membrane to nucleus was observed. Positive rate
was calculated by counting the positive cells in 1000 cells selected randomly in
each slice, and four slices were counted for each stage to get the mean value.
Western blot assay of intracellular
Smad7, CTGF, α-SMA, COL1 protein expression
The confluent cells of the second passage were adjusted to the concentration of
1×105/ml, plated into 25cm2 culture flask. After reaching
confluence, the cells were cultured in 0.1% FCS-F12 and synchronized for 24h.
After reaching the resting stage, the cells were divided into 0, 15min, 30min,
1h and 2h group and incubated with 5ng/ml TGF-β1 for specified time, for
p-Smad2/3 measurements. After removal of supernatant, The cells were washed
twice with PBS, and lysed with protein lysis buffer at 40C for 60min.
centrifugated at 12,000rpm for 15min and the resulting supernatant preserved at
-70oC. 50ug total protein was electrophresed on 10% SDS-PAGE gel and
transferred to nitrocellulose membrane. The membrane was blocked with blocking
buffer containing 3% bovine serum albumin (BSA) and 5% skim milk powder at room
temperature, incubated with anti p-Smad2/3 phosphorylated polyclonal antibody,
Smad7 antibody and COL1 antibody, and the result of immune reaction was detected
with ECL agent.
ELISA assay of FN level
The confluent cells of the second passage were adjusted to the concentration of
1×105/ml, and plated into 24-well culture plate for cell culturing as described
above. After reaching confluence, 0.1% FCS-F12 was added, the cells were
synchronized for 24h and after entering resting stage divided into 0, 24h, 48h,
72h groups and incubated with 5ng/ml TGF-β1 for specified periods of time. The
supernatants were collected and preserved at -20oC. FN in supernatant
content was determined according to the manufacturers' instruction (Shanghai Sun
Biotechnology Inc.).
Detection of Smad7, FN and COL1
intracellular gene expression by RT-PCR
The confluent cells of the second passage were adjusted to the concentration of
1×105/ml, divided into 25cm2 culture flask for cell
culture. After reaching confluence, 0.1% FCS-F12 was added and the cells were
synchronized for 24h. After entering the resting stage the cells were divided
into 0, 24h, 48h, 72h groups and incubated with 5ng/ml TGF-β1 for specified
periods of time. The total RNA was extracted according to the method described
in instruction for Trizol reagent (GibcoBRL Inc., USA). RNA OD values were
determined by ultraviolet spectrophotometer and wavelength pair of 260/280 nm.
OD values ranged from 1.7 to 2.0. Three ribosome RNA strips of 28s, 18s and 5s
were clearly recognized on 1.2% agarose gel electrophoresis, suggesting that no
pollution and degradation took place in total RNA isolation. The first cDNA was
synthesized by reverse transcription according to the instruction for reverse
transcription kit (Promega Inc.), which was then used as a template for PCR
reaction. Primer sequences (synthesized by Shanghai Boya Biotechnology Co. Ltd.)
are shown in Table1![]() .
The reaction condition: pre-denaturation at 950C for 5min, denaturation at 95oC
for 30s, annealing at 56oC for 30s, elongation at 72oC for
50s, followed by 21 cycles for FN and COL1, and 27 cycles for smad7, elongation
at 72oC for 10min, terminated 4oC. The experiments using
the cycles did not yet reach the plateau phase of PCR. PCR products were
isolated by 6% polyacrylamide gel electrophoresis. Gel scanning and analysis was
performed on Gene Genius image analyzer (Syngene Inc). The ratio of optical
density of objective fragment to that of GAPDH fragment acting as control was
used in semi-quantification comparison.
.
The reaction condition: pre-denaturation at 950C for 5min, denaturation at 95oC
for 30s, annealing at 56oC for 30s, elongation at 72oC for
50s, followed by 21 cycles for FN and COL1, and 27 cycles for smad7, elongation
at 72oC for 10min, terminated 4oC. The experiments using
the cycles did not yet reach the plateau phase of PCR. PCR products were
isolated by 6% polyacrylamide gel electrophoresis. Gel scanning and analysis was
performed on Gene Genius image analyzer (Syngene Inc). The ratio of optical
density of objective fragment to that of GAPDH fragment acting as control was
used in semi-quantification comparison.
|
View this table: |
Table1 primer sequence |
Statistical Analysis
Experimental results were expressed as mean ± standard deviation (x±SD). One-way
ANOVA or T test were used for statistical analysis using SPSS11.0 statistical
software package and least significant difference method to analyze the means
further.
|
|
RESULTS
|
|---|
Effect of TGF-β1 on intracellular
The results of immunochemical (Fig.1)![]() were accord with Western blotting's(Fig.2)
were accord with Western blotting's(Fig.2)![]() . The HPMCs hardly expressed p-Smad2/3 protein in the control group and the
cells stained lightly (the positive rate: 3%±1.7%). After TGF-β1 stimulation of
HPMCs Smad2/3 was significantly activated at 15min (the positive rate: 29%±5.8%),
and staining was distributed in cytoplasm; its activity peaked between 30min and
1h (the positive rate: 81%±5.0% and 84%±3.7% respectively). At that time the stain
was deepened and distributed in nucleus or peri-nucleus. The phosphorylation
activity was obviously reduced at 2h (the positive rate: 37%±5.7%), and staining
was lightened and distributed in cytoplasm again. Compared with the control, P
value was less than 0.01.
. The HPMCs hardly expressed p-Smad2/3 protein in the control group and the
cells stained lightly (the positive rate: 3%±1.7%). After TGF-β1 stimulation of
HPMCs Smad2/3 was significantly activated at 15min (the positive rate: 29%±5.8%),
and staining was distributed in cytoplasm; its activity peaked between 30min and
1h (the positive rate: 81%±5.0% and 84%±3.7% respectively). At that time the stain
was deepened and distributed in nucleus or peri-nucleus. The phosphorylation
activity was obviously reduced at 2h (the positive rate: 37%±5.7%), and staining
was lightened and distributed in cytoplasm again. Compared with the control, P
value was less than 0.01.
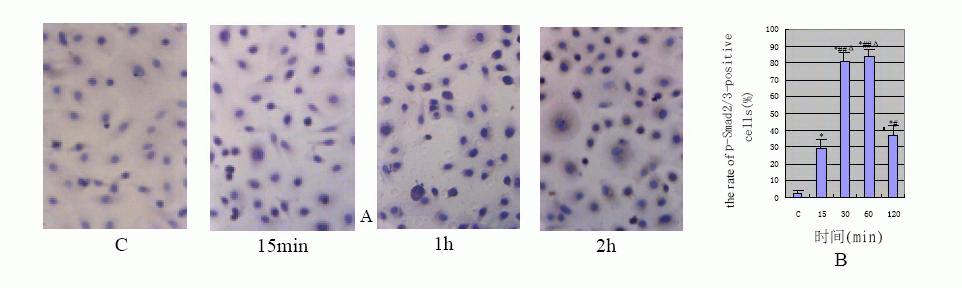
View larger version : |
Fig1 A:the protein expression of p-Smad2/3 displayed by immunohistochemistry (×400); B:TGF-β1-induced the rate of p-Smad2/3-positive cells(*P<0.01 vs C group;#P<0.05,##P<0.01 vs 15 min group;ΔP<0.01 vs 120 min) |

View larger version : |
Fig.2 the protein expression of p-Smad2/3 detected by western blotting |
TGF- β1 effect on Smad7 protein and gene
expression in HPMC
The results of Western blot analysis (Fig.3)![]() the expression of Smad7 in HPMCs was expressed at a low level in control group,
and increased at 24h, peaked at 48h after TGF- β1 stimulation of HPMC, and then
decreased at 72h.
the expression of Smad7 in HPMCs was expressed at a low level in control group,
and increased at 24h, peaked at 48h after TGF- β1 stimulation of HPMC, and then
decreased at 72h.

View larger version : |
Fig.3 Western blot Aanalysis of Smad7 |
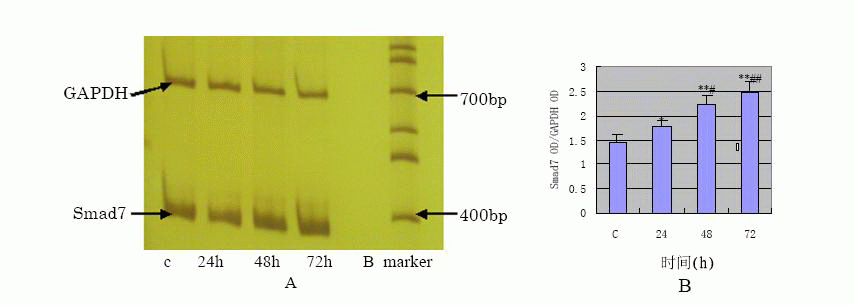
View larger version : |
Fig.4 A:the mRNA expression of Smad7 detected by RT-PCR, B:the semi-quantification result of Smad7 OD/GAPDH OD(*P<0.05, **P<0.01 vs C group; #P<0.05, ##P<0.01 vs 24 h group) |
TGF- β1 effect on FN, COL1 protein and
gene expression in HPMC
The results of Western blot analysis (Fig.5)![]() have shown that COL1 showed very limited expression in the control, increased at
24h in a time-dependent manner after TGF- β1 stimulation of the cells. ELISA
assay showed FN protein expression of supernatants at 24h, 48h, 72h were
2.79±0.44 mg/L 、3.11±0.48 mg/L、 3.55±0.52 mg/L respectively and the results were in
a time-dependent manner compared with the control (0.67±0.07 mg/L and p value
<0.01). The results of semi-quantification RT-PCR of TGF- β1 stimulated HPMC
(Fig.6)
have shown that COL1 showed very limited expression in the control, increased at
24h in a time-dependent manner after TGF- β1 stimulation of the cells. ELISA
assay showed FN protein expression of supernatants at 24h, 48h, 72h were
2.79±0.44 mg/L 、3.11±0.48 mg/L、 3.55±0.52 mg/L respectively and the results were in
a time-dependent manner compared with the control (0.67±0.07 mg/L and p value
<0.01). The results of semi-quantification RT-PCR of TGF- β1 stimulated HPMC
(Fig.6)![]() (Fig.7)
(Fig.7)![]() have shown that mRNA expression of FN and COL1 was increased at 24 h ( p
value<0.01 ) compared with the control, also in a time-dependent manner.
have shown that mRNA expression of FN and COL1 was increased at 24 h ( p
value<0.01 ) compared with the control, also in a time-dependent manner.

View larger version : |
Fig.5 the protein expression of COL1 detected by western blotting |
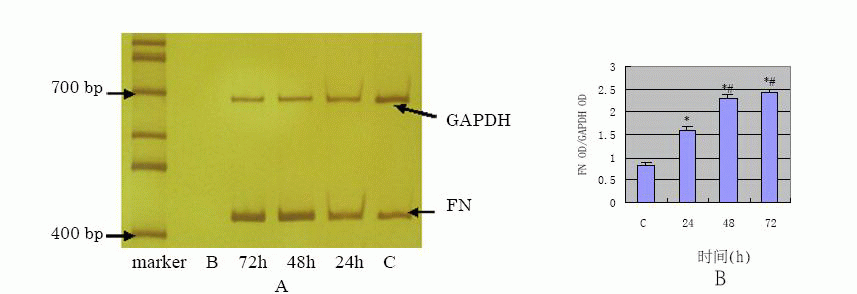
View larger version : |
Fig.6 A:the mRNA expression of FN detected by RT-PCR; B: the semi-quantification result of FN OD/GAPDH OD (*P<0.01 vs C group; #P<0.01 vs 24 h group) |
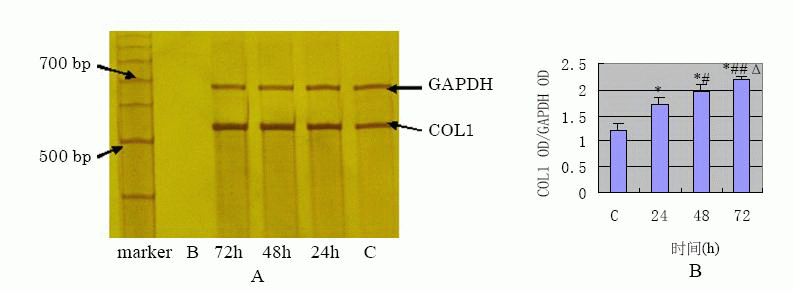
View larger version : |
Fig.7 A:the mRNA expression of FN detected by RT-PCR; B:the semi-quantification result of COL1 OD/GAPDH OD (*P<0.01 vs C group; #P<0.05,##P<0.01 vs 24h group; ΔP<0.05 vs 48h group) |
|
|
|---|
TGF-β1 has been well known as one of the most
important trigger factor in tissue fibrosis. Does TGF-β1 have the same effect on
the peritoneal fibrosis? In this study, when the third passage of HPMCs cells
from its primary cultures were stimulated with TGF-β1, the subsequently obtained
Western blot assay showed that the production of FN and COL1 proteins
significantly increased in a time-dependent manner comparing to the control. The
results of ELISA indicated that FN and COL1 protein levels in supernatants
obtained from TGF-β1-stimulated cells were significantly elevated after 24h of
stimulation. This effect was time-dependent and compared to the control group
significantly elevated at the ρ<0.01. This data are similar to the amplification
data of RT-PCR for FN and COL1 mRNA. The up-regulation of FN and COL1, the major
components of extracellular matrix, demonstrates that ECM components deposit and
because of excessive accumulation result in peritoneal fibrosis. Therefore, our
experiments have demonstrated that TGF-β1 plays an important trigger role in
peritoneal fibrosis.
There have been many researches that have attempted to block the expression or
activity of TGF-β1 from different aspects, in an effort to reduce the
accumulation of extracellular matrix and prevent tissue fibrosis. However,
TGF-β1 which is one of the growth factors with the most complicated function
that have been found so far has a wide range of biological activity on
development, proliferation, differentiation, apoptosis and immune function of a
variety of cells, TGF-β1 has also many side effects such as loss of cell growth
control, immune disturbance, severe inflammatory and even death that will
unavoidably occur if expression or activity of TGF-β1 is to be inhibited as in
treatment of tissue fibrosis As a result, its application in clinical practice
is limited[6]. Recently, with the advances in
TGF-β1 downstream signaling pathway research, the regulatory mechanism of TGF-β1
in tissue fibrosis is gradually being recognized, and inhibition or blockade in
TGF-β1 downstream signaling pathway and its effective mediators have become the
focus of research in prevention of tissue fibrosis.
Smad signaling pathway plays a very important role in TGF-β-induced tissue
fibrosis: (1) SB-431542, a specific blocker for Smad signaling pathway, is able
to suppress the TGF-β1 induced synthesis of extracellular matrix FN and COL1A1
[7], (2) Smad heterogenous oligomer binding site (SBE) exists in
promoters of many genes such as FN, COL1, COL3, COL6, COL7 and COL13, TGF-β can
induce extracellular matrix synthesis through Smad signaling pathway
[3, 4]. Moreover, SBE structure is also found in
promoter of other genes including CTGF [8], TIMP-1
[3], α-SMA [9], PAI-1
[10] and Smad7 [10] which are closely
related to tissue fibrosis,(3) During the course of interaction with other
signaling pathway, Smad pathway is of great importance because other signaling
pathways as it either interveneswith the transmission of Smad pathway or
cooperate with Smad heterogenous oligomer to regulate target gene expression
through its specifically activated transcriptional factor
[11], (4) although little is known about whether specific blockade of
Smad signaling pathway can reduce the side effects caused by direct blockade of
TGF-β> Recent studies suggest that selective blockade of Smad signaling pathway
and p38MAPK pathway by small protein SB-505124 can not only inhibit the
synthesis of extracellular matrix but also prevent the cell death induced by
direct blockade of TGF-β[12].
Does TGF-β1 specially suppress Smad signaling pathway in HPMCs? Smad2/3 is the
receptor-regulated Smads and its activation by phosphorylation is the most
important step in the Smad signaling pathway. Therefore, the expression of
p-Smad2/3 implicates the extent to which Smad signaling pathway is activated
[13]. In our study, it was proven by both immumohistochemical staining
and Western blot assay that the expression of p-Smad2/3 was increased at 15min
after TGF-β1 stimulation, peaking at 1h and simultaneously, the increased
p-Smad2/3 translocated from membrane to cytoplasm then bounded to Smad4 and in
such form entered into nucleus where it exerted the effect of inducing target
genes transcription. Our results confirmed that TGF-β1 could specifically
activate Smad signaling pathway in HPMCs. It was also found in our experiment
that the activation of Smad signaling pathway by TGF-β1 in HPMCs could persist
for long time (at least 2h) and peak at a later timethen the time observed in
renal tubular epithelial cell[13].
Smad7, an inhibitory Smads, negatively regulate Smad signaling pathway[3,
4]. Based on the inhibitory effect of Smad7 on Smad signaling pathway,
studies have been carried out to prevent the tissue fibrosis-induced by TGF-β1
using Smad7 gene transfection. For example, transfected Smad7 gene into normal
rat kidney TEC line (NRK52E cells) and found that overexpression of Smad7
resulted in marked inhibition of TGF- 1 induced Smad2 activation with the
prevention of collagen synthesis[13]. Similarly,
the data of Torada et al indicated that Smad7 gene transfer via adenovirus
electroporation prevents unilateral ureteral obstruction(UUO)-induced renal
fibrosis[14]. In this study, the Western blot
analysis showed that Smad7 protein remained at a low level in the absence of
TGF-β1 but that it was up-regulated remarkably 24h after the stimulation with
TGF-β1, peaking at 48h and persisted for 72h. The result of RT-PCR showed that
the level of Smad7 mRNA was increased in a time-dependent manner with p value
less than 0.05 at 24h and less than 0.01 at both 48 and 72h. These data
demonstrate that a definite level of Smad7 protein is produced by normal HPMCs
and that it act mainly by keeping the Smad signaling pathway from activation.
Once TGF-β1 signaling is initiated, the expression of Smad7 gene is rapidly up
regulated in a feedback manner. However, the feedback is so weak that the
up-regulation of Smad7 cannot offset the TGF-β1-incuded synthesis of FN and
COL1. In our studies, the difference between Smad7 protein production level and
mRNA expression level may be associated with the degradation of Smad7 protein by
obiquitination of activated molecules in Smad signaling pathway[15].
|
|
REFERENCES
|
|---|